当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of γ-Secretase Activation and Substrate Processing.
ACS Central Science ( IF 12.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acscentsci.0c00296 Apurba Bhattarai 1 , Sujan Devkota 1 , Sanjay Bhattarai 1 , Michael S Wolfe 1 , Yinglong Miao 1
ACS Central Science ( IF 12.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acscentsci.0c00296 Apurba Bhattarai 1 , Sujan Devkota 1 , Sanjay Bhattarai 1 , Michael S Wolfe 1 , Yinglong Miao 1
Affiliation
|
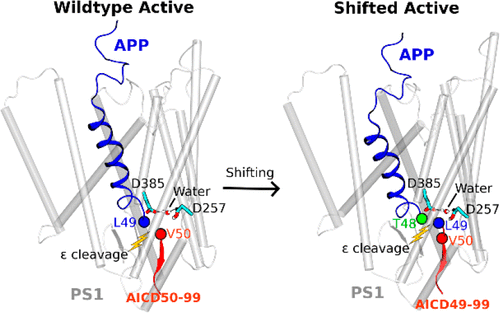
Amyloid β-peptide, the principal component of characteristic cerebral plaques of Alzheimer’s disease (AD), is produced through intramembrane proteolysis of the amyloid precursor protein (APP) by γ-secretase. Despite the importance in the pathogenesis of AD, the mechanisms of intramembrane proteolysis and substrate processing by γ-secretase remain poorly understood. Here, complementary all-atom simulations using a robust Gaussian accelerated molecular dynamics (GaMD) method and biochemical experiments were combined to investigate substrate processing of wildtype and mutant APP by γ-secretase. The GaMD simulations captured spontaneous activation of γ-secretase, with hydrogen bonded catalytic aspartates and water poised for proteolysis of APP at the ε cleavage site. Furthermore, GaMD simulations revealed that familial AD mutations I45F and T48P enhanced the initial ε cleavage between residues Leu49–Val50, while M51F mutation shifted the ε cleavage site to the amide bond between Thr48–Leu49. Detailed analysis of the GaMD simulations allowed us to identify distinct low-energy conformational states of γ-secretase, different secondary structures of the wildtype and mutant APP substrate, and important active-site subpockets for catalytic function of the enzyme. The simulation findings were highly consistent with experimental analyses of APP proteolytic products using mass spectrometry and Western blotting. Taken together, the GaMD simulations and biochemical experiments have enabled us to elucidate the mechanisms of γ-secretase activation and substrate processing, which should facilitate rational computer-aided drug design targeting this functionally important enzyme.
中文翻译:

γ-分泌酶活化和底物加工的机制。
淀粉样蛋白β肽是阿尔茨海默氏病(AD)的特征性大脑斑块的主要成分,是由γ分泌酶通过淀粉样蛋白前体蛋白(APP)的膜内蛋白水解产生的。尽管在AD的发病机理中很重要,但是对膜内蛋白水解和γ-分泌酶对底物的加工机理仍知之甚少。在这里,结合使用健壮的高斯加速分子动力学(GaMD)方法和生物化学实验的互补全原子模拟,以研究野生型和突变体APP通过γ-分泌酶的底物加工。GaMD模拟捕获了γ-分泌酶的自发激活,其中氢键结合的催化天冬氨酸和水准备在ε裂解位点进行APP的蛋白水解。此外,GaMD模拟显示,家族性AD突变I45F和T48P增强了残基Leu49–Val50之间的初始ε裂解,而M51F突变则将ε裂解位点转移至Thr48–Leu49之间的酰胺键。GaMD模拟的详细分析使我们能够确定γ-分泌酶的不同低能构象状态,野生型和突变APP底物的不同二级结构,以及该酶催化功能的重要活性位点。模拟结果与使用质谱和蛋白质印迹的APP蛋白水解产物的实验分析高度一致。综上所述,GaMD模拟和生化实验使我们能够阐明γ-分泌酶激活和底物加工的机制,
更新日期:2020-06-24
中文翻译:

γ-分泌酶活化和底物加工的机制。
淀粉样蛋白β肽是阿尔茨海默氏病(AD)的特征性大脑斑块的主要成分,是由γ分泌酶通过淀粉样蛋白前体蛋白(APP)的膜内蛋白水解产生的。尽管在AD的发病机理中很重要,但是对膜内蛋白水解和γ-分泌酶对底物的加工机理仍知之甚少。在这里,结合使用健壮的高斯加速分子动力学(GaMD)方法和生物化学实验的互补全原子模拟,以研究野生型和突变体APP通过γ-分泌酶的底物加工。GaMD模拟捕获了γ-分泌酶的自发激活,其中氢键结合的催化天冬氨酸和水准备在ε裂解位点进行APP的蛋白水解。此外,GaMD模拟显示,家族性AD突变I45F和T48P增强了残基Leu49–Val50之间的初始ε裂解,而M51F突变则将ε裂解位点转移至Thr48–Leu49之间的酰胺键。GaMD模拟的详细分析使我们能够确定γ-分泌酶的不同低能构象状态,野生型和突变APP底物的不同二级结构,以及该酶催化功能的重要活性位点。模拟结果与使用质谱和蛋白质印迹的APP蛋白水解产物的实验分析高度一致。综上所述,GaMD模拟和生化实验使我们能够阐明γ-分泌酶激活和底物加工的机制,











































 京公网安备 11010802027423号
京公网安备 11010802027423号