当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Solvent-Exposed Fe-S D-Cluster Contributes to Oxygen-Resistance in Desulfovibrio vulgaris Ni-Fe Carbon Monoxide Dehydrogenase.
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-04 , DOI: 10.1021/acscatal.0c00934 Elizabeth C Wittenborn 1 , Chloé Guendon 2 , Mériem Merrouch 2 , Martino Benvenuti 2 , Vincent Fourmond 2 , Christophe Léger 2 , Catherine L Drennan 1, 1, 1, 3 , Sébastien Dementin 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-04 , DOI: 10.1021/acscatal.0c00934 Elizabeth C Wittenborn 1 , Chloé Guendon 2 , Mériem Merrouch 2 , Martino Benvenuti 2 , Vincent Fourmond 2 , Christophe Léger 2 , Catherine L Drennan 1, 1, 1, 3 , Sébastien Dementin 2
Affiliation
|
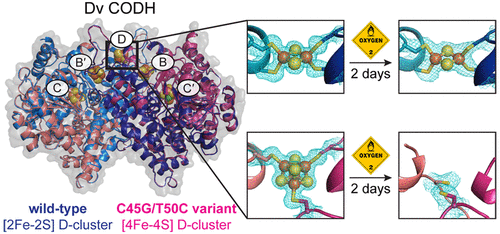
Ni–Fe CO-dehydrogenases (CODHs) catalyze the conversion between CO and CO2 using a chain of Fe–S clusters to mediate long-range electron transfer. One of these clusters, the D-cluster, is surface-exposed and serves to transfer electrons between CODH and external redox partners. These enzymes tend to be extremely O2-sensitive and are always manipulated under strictly anaerobic conditions. However, the CODH from Desulfovibrio vulgaris (Dv) appears unique: exposure to micromolar concentrations of O2 on the minutes-time scale only reversibly inhibits the enzyme, and full activity is recovered after reduction. Here, we examine whether this unusual property of Dv CODH results from the nature of its D-cluster, which is a [2Fe-2S] cluster, instead of the [4Fe-4S] cluster observed in all other characterized CODHs. To this aim, we produced and characterized a Dv CODH variant where the [2Fe-2S] D-cluster is replaced with a [4Fe-4S] D-cluster through mutagenesis of the D-cluster–binding sequence motif. We determined the crystal structure of this CODH variant to 1.83-Å resolution and confirmed the incorporation of a [4Fe-4S] D-cluster. We show that upon long-term O2-exposure, the [4Fe-4S] D-cluster degrades, whereas the [2Fe-2S] D-cluster remains intact. Crystal structures of the Dv CODH variant exposed to O2 for increasing periods of time provide snapshots of [4Fe-4S] D-cluster degradation. We further show that the WT enzyme purified under aerobic conditions retains 30% activity relative to a fully anaerobic purification, compared to 10% for the variant, and the WT enzyme loses activity more slowly than the variant upon prolonged aerobic storage. The D-cluster is therefore a key site of irreversible oxidative damage in Dv CODH, and the presence of a [2Fe-2S] D-cluster contributes to the O2-tolerance of this enzyme. Together, these results relate O2-sensitivity with the details of the protein structure in this family of enzymes.
中文翻译:

溶剂暴露的Fe-S D团簇有助于寻常脱硫弧菌Ni-Fe一氧化碳脱氢酶中的抗氧性。
Ni-Fe CO-脱氢酶(CODHs)使用一串Fe-S簇来介导长距离电子转移,催化CO和CO 2之间的转化。这些簇之一,D簇,是表面暴露的,用于在CODH和外部氧化还原伙伴之间转移电子。这些酶往往对O 2极为敏感,并且始终在严格的厌氧条件下进行操作。但是,寻常脱硫弧菌(Dv)的CODH似乎很独特:暴露于微摩尔浓度的O 2以分钟为单位的时间仅可逆地抑制酶,还原后可以恢复全部活性。在这里,我们检查Dv CODH的这种异常性质是否是由于其D簇的性质所致,它是[2Fe-2S]簇,而不是在所有其他特征性CODH中观察到的[4Fe-4S]簇。为此,我们生产并鉴定了一种Dv CODH变体,其中通过诱变D-簇结合序列基序,将[2Fe-2S] D-簇替换为[4Fe-4S] D-簇。我们确定了该CODH变体的晶体结构至1.83Å的分辨率,并证实了[4Fe-4S] D团簇的结合。我们显示,长期暴露于O 2-时,[4Fe-4S] D群集会降解,而[2Fe-2S] D群集则保持完整。Dv CODH变体暴露于O的晶体结构2为增加的时间段提供[4Fe-4S] D群集退化的快照。我们进一步表明,在有氧条件下纯化的WT酶相对于完全厌氧纯化保留30%的活性,而对于变体则为10%,并且在长期需氧存储下WT酶的活性比变种更慢。因此,D簇是Dv CODH中不可逆氧化损伤的关键部位,[2Fe-2S] D簇的存在有助于该酶的O 2耐受性。总之,这些结果将O 2敏感性与该酶家族中蛋白质结构的细节联系起来。
更新日期:2020-07-02
中文翻译:

溶剂暴露的Fe-S D团簇有助于寻常脱硫弧菌Ni-Fe一氧化碳脱氢酶中的抗氧性。
Ni-Fe CO-脱氢酶(CODHs)使用一串Fe-S簇来介导长距离电子转移,催化CO和CO 2之间的转化。这些簇之一,D簇,是表面暴露的,用于在CODH和外部氧化还原伙伴之间转移电子。这些酶往往对O 2极为敏感,并且始终在严格的厌氧条件下进行操作。但是,寻常脱硫弧菌(Dv)的CODH似乎很独特:暴露于微摩尔浓度的O 2以分钟为单位的时间仅可逆地抑制酶,还原后可以恢复全部活性。在这里,我们检查Dv CODH的这种异常性质是否是由于其D簇的性质所致,它是[2Fe-2S]簇,而不是在所有其他特征性CODH中观察到的[4Fe-4S]簇。为此,我们生产并鉴定了一种Dv CODH变体,其中通过诱变D-簇结合序列基序,将[2Fe-2S] D-簇替换为[4Fe-4S] D-簇。我们确定了该CODH变体的晶体结构至1.83Å的分辨率,并证实了[4Fe-4S] D团簇的结合。我们显示,长期暴露于O 2-时,[4Fe-4S] D群集会降解,而[2Fe-2S] D群集则保持完整。Dv CODH变体暴露于O的晶体结构2为增加的时间段提供[4Fe-4S] D群集退化的快照。我们进一步表明,在有氧条件下纯化的WT酶相对于完全厌氧纯化保留30%的活性,而对于变体则为10%,并且在长期需氧存储下WT酶的活性比变种更慢。因此,D簇是Dv CODH中不可逆氧化损伤的关键部位,[2Fe-2S] D簇的存在有助于该酶的O 2耐受性。总之,这些结果将O 2敏感性与该酶家族中蛋白质结构的细节联系起来。











































 京公网安备 11010802027423号
京公网安备 11010802027423号