当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Advancing physiological maturation in human iPSC-derived cardiac muscle by gene editing an inducible adult troponin isoform switch
STEM CELLS ( IF 5.2 ) Pub Date : 2020-06-16 , DOI: 10.1002/stem.3235 Matthew Wheelwright 1 , Jennifer Mikkila 1 , Fikru B Bedada 1 , Mohammad A Mandegar 2 , Brian R Thompson 1 , Joseph M Metzger 1
STEM CELLS ( IF 5.2 ) Pub Date : 2020-06-16 , DOI: 10.1002/stem.3235 Matthew Wheelwright 1 , Jennifer Mikkila 1 , Fikru B Bedada 1 , Mohammad A Mandegar 2 , Brian R Thompson 1 , Joseph M Metzger 1
Affiliation
|
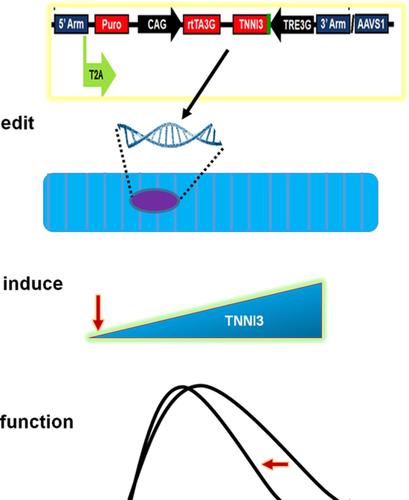
Advancing maturation of stem cell‐derived cardiac muscle represents a major barrier to progress in cardiac regenerative medicine. Cardiac muscle maturation involves a myriad of gene, protein, and cell‐based transitions, spanning across all aspects of cardiac muscle form and function. We focused here on a key developmentally controlled transition in the cardiac sarcomere, the functional unit of the heart. Using a gene‐editing platform, human induced pluripotent stem cell (hiPSCs) were engineered with a drug‐inducible expression cassette driving the adult cardiac troponin I (cTnI) regulatory isoform, a transition shown to be a rate‐limiting step in advancing sarcomeric maturation of hiPSC cardiac muscle (hiPSC‐CM) toward the adult state. Findings show that induction of the adult cTnI isoform resulted in the physiological acquisition of adult‐like cardiac contractile function in hiPSC‐CMs in vitro. Specifically, cTnI induction accelerated relaxation kinetics at baseline conditions, a result independent of alterations in the kinetics of the intracellular Ca2+ transient. In comparison, isogenic unedited hiPSC‐CMs had no cTnI induction and no change in relaxation function. Temporal control of adult cTnI isoform induction did not alter other developmentally regulated sarcomere transitions, including myosin heavy chain isoform expression, nor did it affect expression of SERCA2a or phospholamban. Taken together, precision genetic targeting of sarcomere maturation via inducible TnI isoform switching enables physiologically relevant adult myocardium‐like contractile adaptations that are essential for beat‐to‐beat modulation of adult human heart performance. These findings have relevance to hiPSC‐CM structure‐function and drug‐discovery studies in vitro, as well as for potential future clinical applications of physiologically optimized hiPSC‐CM in cardiac regeneration/repair.
中文翻译:

通过基因编辑诱导型成人肌钙蛋白同工型开关促进人 iPSC 衍生心肌的生理成熟
促进干细胞衍生心肌的成熟是心脏再生医学进展的主要障碍。心肌成熟涉及无数基于基因、蛋白质和细胞的转变,跨越心肌形式和功能的所有方面。我们在这里重点关注心脏肌节(心脏的功能单位)中一个关键的发育控制转变。使用基因编辑平台,人类诱导多能干细胞 (hiPSCs) 被设计成具有驱动成人心肌肌钙蛋白 I (cTnI) 调节异构体的药物诱导表达盒,这一转变被证明是促进肌节成熟的限速步骤hiPSC 心肌 (hiPSC-CM) 向成年状态的变化。研究结果表明,成人 cTnI 异构体的诱导导致体外 hiPSC-CM 中成人样心脏收缩功能的生理获得。具体而言,cTnI 诱导加速了基线条件下的松弛动力学,其结果与细胞内 Ca2+ 瞬变动力学的改变无关。相比之下,同基因未编辑的 hiPSC-CM 没有 cTnI 诱导,松弛功能也没有变化。成人 cTnI 异构体诱导的时间控制不会改变其他发育调控的肌节转变,包括肌球蛋白重链异构体的表达,也不会影响 SERCA2a 或磷蛋白的表达。综合起来,通过可诱导的 TnI 异构体转换对肌节成熟的精确基因靶向使生理相关的成人心肌样收缩适应成为可能,这对于成人心脏功能的逐搏调节至关重要。这些发现与 hiPSC-CM 结构-功能和体外药物发现研究有关,以及生理优化的 hiPSC-CM 在心脏再生/修复中的潜在未来临床应用。
更新日期:2020-06-16
中文翻译:

通过基因编辑诱导型成人肌钙蛋白同工型开关促进人 iPSC 衍生心肌的生理成熟
促进干细胞衍生心肌的成熟是心脏再生医学进展的主要障碍。心肌成熟涉及无数基于基因、蛋白质和细胞的转变,跨越心肌形式和功能的所有方面。我们在这里重点关注心脏肌节(心脏的功能单位)中一个关键的发育控制转变。使用基因编辑平台,人类诱导多能干细胞 (hiPSCs) 被设计成具有驱动成人心肌肌钙蛋白 I (cTnI) 调节异构体的药物诱导表达盒,这一转变被证明是促进肌节成熟的限速步骤hiPSC 心肌 (hiPSC-CM) 向成年状态的变化。研究结果表明,成人 cTnI 异构体的诱导导致体外 hiPSC-CM 中成人样心脏收缩功能的生理获得。具体而言,cTnI 诱导加速了基线条件下的松弛动力学,其结果与细胞内 Ca2+ 瞬变动力学的改变无关。相比之下,同基因未编辑的 hiPSC-CM 没有 cTnI 诱导,松弛功能也没有变化。成人 cTnI 异构体诱导的时间控制不会改变其他发育调控的肌节转变,包括肌球蛋白重链异构体的表达,也不会影响 SERCA2a 或磷蛋白的表达。综合起来,通过可诱导的 TnI 异构体转换对肌节成熟的精确基因靶向使生理相关的成人心肌样收缩适应成为可能,这对于成人心脏功能的逐搏调节至关重要。这些发现与 hiPSC-CM 结构-功能和体外药物发现研究有关,以及生理优化的 hiPSC-CM 在心脏再生/修复中的潜在未来临床应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号