当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemically Generated Interfacial pH Change: Application to Signal‐Triggered Molecule Release
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-06-05 , DOI: 10.1002/celc.202000615 Paolo Bollella 1 , Artem Melman 1 , Evgeny Katz 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-06-05 , DOI: 10.1002/celc.202000615 Paolo Bollella 1 , Artem Melman 1 , Evgeny Katz 1
Affiliation
|
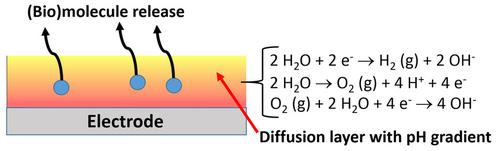
Electron transfer processes during redox reactions are frequently accompanied with protonation/deprotonation processes, thus changing H+/OH− concentrations. When the redox reactions proceed at electrode surfaces, being electrochemically processed, changes in local interfacial pH are possible, particularly when the electrolyte solution is not strongly buffered. The pH gradient can be produced in the diffusion layer and its thickness depends on the rate of electrochemical process, which is measured as the current density, diffusion rates, and buffer capacity. While conventional techniques for measuring pH values are not applicable to a very thin diffusional layer, special methods have been developed for the interfacial pH measurements, including scanning electrochemical microscopy (SECM), rotating ring‐disk electrode (RRDE) measurements, various optical methods, particularly using confocal fluorescent microscopy, and others. Dramatic pH changes proceeding in a near‐electrode layer have been reported for H2O reduction/oxidation, O2 reduction, and some other electrochemical electron/proton transfer processes. These pH changes can be used to trigger some other physical processes, particularly (bio)molecule release processes from modified electrodes, which can be destabilized upon the electrochemically generated pH changes, thus releasing entrapped/loaded target molecules. This review‐type article overviews the formation and analysis of locally produced interfacial pH changes and their use for electrochemically triggered (bio)molecule release. The paper briefly reviews the research area, then concentrating on the systems designed recently by the authors.
中文翻译:

电化学产生的界面pH值变化:在信号触发的分子释放中的应用
期间的氧化还原反应的电子传递过程经常伴随着质子化/去质子化处理,从而改变ħ + / OH -浓度。当氧化还原反应在经过电化学处理的电极表面上进行时,局部界面pH值可能会发生变化,特别是当电解质溶液未充分缓冲时。可以在扩散层中产生pH梯度,其厚度取决于电化学过程的速率,可以通过电流密度,扩散速率和缓冲容量来衡量。尽管传统的pH值测量技术不适用于非常薄的扩散层,但已经为界面pH测量开发了特殊方法,包括扫描电化学显微镜(SECM),旋转圆盘电极(RRDE)测量,各种光学方法,特别是使用共聚焦荧光显微镜等。2 O还原/氧化,O 2还原和其他一些电化学电子/质子转移过程。这些pH变化可用于触发其他一些物理过程,特别是从修饰电极释放(生物)分子的过程,这些过程在电化学产生的pH变化时会不稳定,从而释放出被包裹/负载的目标分子。这篇评论类型的文章概述了局部产生的界面pH变化的形成和分析,以及它们在电化学触发的(生物)分子释放中的用途。本文简要回顾了研究领域,然后重点介绍了作者最近设计的系统。
更新日期:2020-06-05
中文翻译:

电化学产生的界面pH值变化:在信号触发的分子释放中的应用
期间的氧化还原反应的电子传递过程经常伴随着质子化/去质子化处理,从而改变ħ + / OH -浓度。当氧化还原反应在经过电化学处理的电极表面上进行时,局部界面pH值可能会发生变化,特别是当电解质溶液未充分缓冲时。可以在扩散层中产生pH梯度,其厚度取决于电化学过程的速率,可以通过电流密度,扩散速率和缓冲容量来衡量。尽管传统的pH值测量技术不适用于非常薄的扩散层,但已经为界面pH测量开发了特殊方法,包括扫描电化学显微镜(SECM),旋转圆盘电极(RRDE)测量,各种光学方法,特别是使用共聚焦荧光显微镜等。2 O还原/氧化,O 2还原和其他一些电化学电子/质子转移过程。这些pH变化可用于触发其他一些物理过程,特别是从修饰电极释放(生物)分子的过程,这些过程在电化学产生的pH变化时会不稳定,从而释放出被包裹/负载的目标分子。这篇评论类型的文章概述了局部产生的界面pH变化的形成和分析,以及它们在电化学触发的(生物)分子释放中的用途。本文简要回顾了研究领域,然后重点介绍了作者最近设计的系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号