Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
GLAST-CreERT2 mediated deletion of GDNF increases brain damage and exacerbates long-term stroke outcomes after focal ischemic stroke in mouse model.
Glia ( IF 5.4 ) Pub Date : 2020-06-04 , DOI: 10.1002/glia.23848 Nannan Zhang 1 , Zhe Zhang 2 , Rui He 1 , Hailong Li 1, 2 , Shinghua Ding 1, 2
Glia ( IF 5.4 ) Pub Date : 2020-06-04 , DOI: 10.1002/glia.23848 Nannan Zhang 1 , Zhe Zhang 2 , Rui He 1 , Hailong Li 1, 2 , Shinghua Ding 1, 2
Affiliation
|
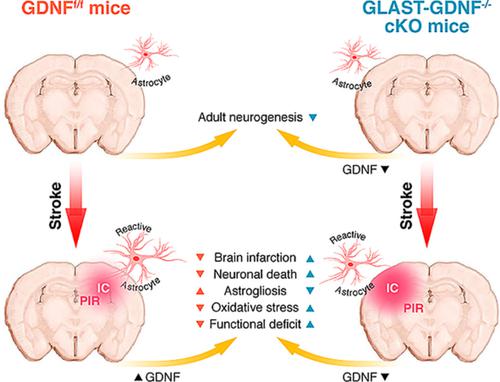
Focal ischemic stroke (FIS) is a leading cause of human death. Glial scar formation largely caused by reactive astrogliosis in peri‐infarct region (PIR) is the hallmark of FIS. Glial cell‐derived neurotrophic factor (GDNF) was originally isolated from a rat glioma cell‐line supernatant and is a potent survival neurotrophic factor. Here, using CreERT2–LoxP recombination technology, we generated inducible and astrocyte‐specific GDNF conditional knockout (cKO), that is, GLAST‐GDNF−/− cKO mice to investigate the effect of reactive astrocytes (RAs)‐derived GDNF on neuronal death, brain damage, oxidative stress and motor function recovery after photothrombosis (PT)‐induced FIS. Under non‐ischemic conditions, we found that adult GLAST‐GDNF−/− cKO mice exhibited significant lower numbers of Brdu+, Ki67+ cells, and DCX+ cells in the dentate gyrus (DG) in hippocampus than GDNF floxed (GDNFf/f) control (Ctrl) mice, indicating endogenous astrocytic GDNF can promote adult neurogenesis. Under ischemic conditions, GLAST‐GDNF−/− cKO mice had a significant increase in infarct volume, hippocampal damage and FJB+ degenerating neurons after PT as compared with the Ctrl mice. GLAST‐GDNF−/− cKO mice also had lower densities of Brdu+ and Ki67+ cells in the PIR and exhibited larger behavioral deficits than the Ctrl mice. Mechanistically, GDNF deficiency in astrocytes increased oxidative stress through the downregulation of glucose‐6‐phosphate dehydrogenase (G6PD) in RAs. In summary, our study indicates that RAs‐derived endogenous GDNF plays important roles in reducing brain damage and promoting brain recovery after FIS through neural regeneration and suggests that promoting anti‐oxidant mechanism in RAs is a potential strategy in stroke therapy.
中文翻译:

在小鼠模型中,GLAST-CreERT2 介导的 GDNF 缺失会增加脑损伤并加剧局灶性缺血性卒中后的长期卒中结果。
局灶性缺血性中风 (FIS) 是导致人类死亡的主要原因。主要由梗死周围区域 (PIR) 的反应性星形胶质细胞增生引起的胶质瘢痕形成是 FIS 的标志。神经胶质细胞衍生的神经营养因子 (GDNF) 最初是从大鼠神经胶质瘤细胞系上清液中分离出来的,是一种有效的存活神经营养因子。在这里,我们使用CreER T2 –LoxP重组技术,生成了诱导型和星形胶质细胞特异性 GDNF 条件敲除 (cKO),即 GLAST-GDNF -/- cKO 小鼠,以研究反应性星形胶质细胞 (RAs) 衍生的 GDNF 对神经元的影响光血栓形成(PT)诱导的 FIS 后死亡、脑损伤、氧化应激和运动功能恢复。在非缺血条件下,我们发现成人 GLAST-GDNF -/-cKO 小鼠海马齿状回 (DG) 中 Brdu+、Ki67+ 细胞和 DCX+ 细胞的数量显着低于 GDNF floxed (GDNF f/f ) 对照 (Ctrl) 小鼠,表明内源性星形胶质细胞 GDNF 可以促进成体神经发生。在缺血条件下,与 Ctrl 小鼠相比,GLAST-GDNF -/- cKO 小鼠在 PT 后梗死体积、海马损伤和 FJB+ 退化神经元显着增加。GLAST-GDNF -/-cKO 小鼠在 PIR 中的 Brdu+ 和 Ki67+ 细胞密度也较低,并且表现出比 Ctrl 小鼠更大的行为缺陷。从机制上讲,星形胶质细胞中的 GDNF 缺乏通过下调 RA 中的葡萄糖 6-磷酸脱氢酶 (G6PD) 来增加氧化应激。总之,我们的研究表明,RAs 衍生的内源性 GDNF 通过神经再生在减少脑损伤和促进 FIS 后脑恢复方面发挥重要作用,并表明促进 RA 中的抗氧化机制是卒中治疗的潜在策略。
更新日期:2020-06-04
中文翻译:

在小鼠模型中,GLAST-CreERT2 介导的 GDNF 缺失会增加脑损伤并加剧局灶性缺血性卒中后的长期卒中结果。
局灶性缺血性中风 (FIS) 是导致人类死亡的主要原因。主要由梗死周围区域 (PIR) 的反应性星形胶质细胞增生引起的胶质瘢痕形成是 FIS 的标志。神经胶质细胞衍生的神经营养因子 (GDNF) 最初是从大鼠神经胶质瘤细胞系上清液中分离出来的,是一种有效的存活神经营养因子。在这里,我们使用CreER T2 –LoxP重组技术,生成了诱导型和星形胶质细胞特异性 GDNF 条件敲除 (cKO),即 GLAST-GDNF -/- cKO 小鼠,以研究反应性星形胶质细胞 (RAs) 衍生的 GDNF 对神经元的影响光血栓形成(PT)诱导的 FIS 后死亡、脑损伤、氧化应激和运动功能恢复。在非缺血条件下,我们发现成人 GLAST-GDNF -/-cKO 小鼠海马齿状回 (DG) 中 Brdu+、Ki67+ 细胞和 DCX+ 细胞的数量显着低于 GDNF floxed (GDNF f/f ) 对照 (Ctrl) 小鼠,表明内源性星形胶质细胞 GDNF 可以促进成体神经发生。在缺血条件下,与 Ctrl 小鼠相比,GLAST-GDNF -/- cKO 小鼠在 PT 后梗死体积、海马损伤和 FJB+ 退化神经元显着增加。GLAST-GDNF -/-cKO 小鼠在 PIR 中的 Brdu+ 和 Ki67+ 细胞密度也较低,并且表现出比 Ctrl 小鼠更大的行为缺陷。从机制上讲,星形胶质细胞中的 GDNF 缺乏通过下调 RA 中的葡萄糖 6-磷酸脱氢酶 (G6PD) 来增加氧化应激。总之,我们的研究表明,RAs 衍生的内源性 GDNF 通过神经再生在减少脑损伤和促进 FIS 后脑恢复方面发挥重要作用,并表明促进 RA 中的抗氧化机制是卒中治疗的潜在策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号