当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bis-Selenoureas for Anion Binding: A 1H NMR and Theoretical Study.
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-06-04 , DOI: 10.1002/cplu.202000260 Giacomo Picci 1 , Rita Mocci 1 , Gianluca Ciancaleoni 2 , Vito Lippolis 1 , Mariola Zielińska-Błajet 3 , Claudia Caltagirone 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-06-04 , DOI: 10.1002/cplu.202000260 Giacomo Picci 1 , Rita Mocci 1 , Gianluca Ciancaleoni 2 , Vito Lippolis 1 , Mariola Zielińska-Błajet 3 , Claudia Caltagirone 1
Affiliation
|
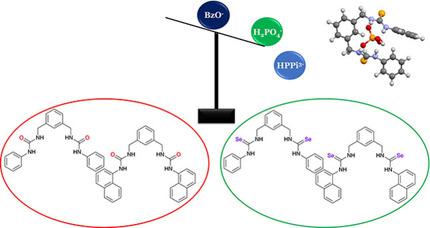
The anion binding ability of a family of bis‐selenoureas L1 ‐L3 obtained by the reaction of 1,3‐bis(aminomethyl)‐benzene and phenylisoselenocyanate, p ‐methoxyphenylisoselenocyanate and naphtylisoselenocyanate, for L1 , L2 , and L3 , respectively, has been tested and compared to that of previously described bis‐urea analogues. Results suggest that the introduction of selenium leads to an increase in the acidity of the urea NH hydrogen atoms, and therefore to a stronger affinity (more than three‐fold higher) towards anion species, in particular dihydrogen phosphate, in DMSO‐d 6. Theoretical calculations allowed for the optimization of the adducts receptors corroborating the experimental results.
中文翻译:

用于阴离子结合的双硒代脲:1H NMR和理论研究。
一个家庭的阴离子结合能力双selenoureas L1 - L3由1,3-双(氨基甲基) -苯和phenylisoselenocyanate,反应得到p -methoxyphenylisoselenocyanate和naphtylisoselenocyanate,为L1,L2,和L3,分别,一直测试并与先前描述的双脲类似物进行比较。结果表明,硒的引入导致尿素NH氢原子的酸度增加,因此对DMSO- d 6中的阴离子种类,特别是磷酸二氢盐具有更强的亲和力(高三倍以上)。。理论计算可以优化加合物受体,从而证实了实验结果。
更新日期:2020-07-01
中文翻译:

用于阴离子结合的双硒代脲:1H NMR和理论研究。
一个家庭的阴离子结合能力双selenoureas L1 - L3由1,3-双(氨基甲基) -苯和phenylisoselenocyanate,反应得到p -methoxyphenylisoselenocyanate和naphtylisoselenocyanate,为L1,L2,和L3,分别,一直测试并与先前描述的双脲类似物进行比较。结果表明,硒的引入导致尿素NH氢原子的酸度增加,因此对DMSO- d 6中的阴离子种类,特别是磷酸二氢盐具有更强的亲和力(高三倍以上)。。理论计算可以优化加合物受体,从而证实了实验结果。









































 京公网安备 11010802027423号
京公网安备 11010802027423号