当前位置:
X-MOL 学术
›
Miner. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational insights into the adsorption mechanism of gallic acid-bearing reagents on calcium-bearing mineral surfaces
Minerals Engineering ( IF 4.9 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.mineng.2020.106485 Jianyong He , Yuehua Hu , Wei Sun , Chenyang Zhang , Chenhu Zhang , Shangyong Lin , Jingxiang Zou
Minerals Engineering ( IF 4.9 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.mineng.2020.106485 Jianyong He , Yuehua Hu , Wei Sun , Chenyang Zhang , Chenhu Zhang , Shangyong Lin , Jingxiang Zou
|
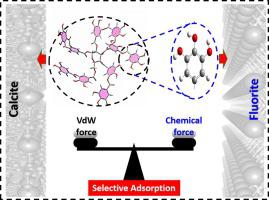
Abstract Interaction mechanisms of flotation reagents including pyrogallic acid (pyGA), GA and tannic acid (TA) adsorption onto fluorite (1 0 0) and calcite (1 0 4) surfaces were investigated by first-principles calculations, molecular mechanics (MM) and molecular dynamics (MD) approaches under vacuum phases to understand the spatial effects of molecules. First-principles calculations indicated that GA/pyGA could more strongly adsorb onto the fluorite surface than onto the calcite surface, but MM and MD results indicated TA tended to adsorb onto the calcite surface rather than the fluorite surface. These results were consistent with reported flotation results. Further analysis showed that the interaction difference was ascribed to the different balance of the corresponding chemical and van der Waals forces on different mineral surfaces.
中文翻译:

含没食子酸试剂在含钙矿物表面吸附机制的计算洞察
摘要 通过第一性原理计算、分子力学(MM)和方解石(1 0 4)表面吸附焦没食子酸(pyGA)、GA和单宁酸(TA)等浮选试剂的相互作用机理进行了研究。真空相下的分子动力学 (MD) 方法以了解分子的空间效应。第一性原理计算表明,GA/pyGA 在萤石表面的吸附能力强于方解石表面,但 MM 和 MD 结果表明 TA 倾向于吸附在方解石表面而不是萤石表面。这些结果与报道的浮选结果一致。进一步分析表明,相互作用的差异归因于不同矿物表面上相应化学力和范德华力的不同平衡。
更新日期:2020-09-01
中文翻译:

含没食子酸试剂在含钙矿物表面吸附机制的计算洞察
摘要 通过第一性原理计算、分子力学(MM)和方解石(1 0 4)表面吸附焦没食子酸(pyGA)、GA和单宁酸(TA)等浮选试剂的相互作用机理进行了研究。真空相下的分子动力学 (MD) 方法以了解分子的空间效应。第一性原理计算表明,GA/pyGA 在萤石表面的吸附能力强于方解石表面,但 MM 和 MD 结果表明 TA 倾向于吸附在方解石表面而不是萤石表面。这些结果与报道的浮选结果一致。进一步分析表明,相互作用的差异归因于不同矿物表面上相应化学力和范德华力的不同平衡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号