Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-06-05 , DOI: 10.1016/j.jfluchem.2020.109587 E. Goreshnik , Z. Mazej
|
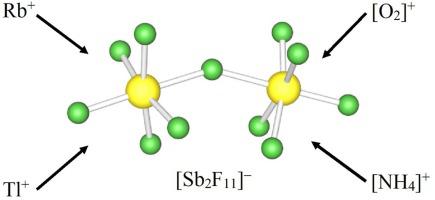
The crystal structures of RbSb2F11, TlSb2F11, O2Sb2F11 and β-NH4Sb2F11 salts are isotypical and crystallize in the orthorhombic space group Cmc21 (No. 36). Crystal data of rubidium salt: a = 19.9981(3) Å, b = 11.5093(2) Å, c = 12.9623(2) Å, V = 2983.46(8) Å3, and Z = 12 at 150 K. Unit cell parameters of corresponding thallium salt: a = 19.9876(5) Å, b = 11.5400(3) Å, c = 13.0267(3) Å, V = 3004.70(13) Å3 and Z = 12 at 150 K. Reaction between NH4F and excess of SbF5 in anhydrous HF resulted in the single-crystal growth of β-NH4Sb2F11: a = 20.0406(7) Å, b = 11.5534(4) Å, c = 13.0796(4) Å, V = 3028.42(18) Å3, and Z = 12 at 296 K. The XRD experiments on NH4Sb2F11 at 240 and 150 K revealed another modification (α-NH4Sb2F11), which crystallizes in a (pseudo-hexagonal) monoclinic cell, space group P21, a = 11.5045(6) Å, b = 13.0437(3) Å, c = 11.5264(6) Å, β = 119.818(7)°, V = 1500.67(15) Å3, and Z = 6 at 240 K. Reaction between the O2SbF6 and SbF5 in anhydrous HF yielded O2Sb2F11 upon crystallization with a = 19.8472(5) Å, b = 11.1172(3) Å, c = 12.8259(3) Å, V = 2829.97(12) Å3, and Z = 12 at 150 K. The crystal structures of ASb2F11 (A = Rb+, Tl+, O2+, NH4+) salts consist of dimeric Sb2F11− anions with partially disordered terminal fluorine atoms. Negative charge of the anions is compensated by single charged cations. Crystal structure of α-NH4Sb2F11 demonstrates similar motif as in β-phase but with slightly re-arranged N―H… F hydrogen bonds. In the 100−296 K range, only rhombohedral phase was observed for NH4SbF6 (rhombohedral space group R , No. 148) with a = b = 7.5961(9) Å, c = 7.7176(9) Å, α = β = 90°, γ = 120°, V = 385.65(12) Å3, and Z = 3 at 150 K).
中文翻译:

同型ASb 2 F 11(A = Rb +,Tl +,O 2 +)和β- NH 4 Sb 2 F 11的晶体结构以及低温α - NH 4 Sb 2 F 11的晶体结构
RbSb 2 F 11,TlSb 2 F 11,O 2 Sb 2 F 11和β- NH 4 Sb 2 F 11盐的晶体结构是同型的,并且在正交晶空间群Cmc2 1(第36号)中结晶。铷盐的晶体数据:一个 = 19.9981(3)埃,b = 11.5093(2)埃,c ^ = 12.9623(2),V = 2983.46(8)埃3,与ž = 12个,在150个K.晶胞参数对应铊盐:一个 = 19.9876(5),b = 11.5400(3)埃,c ^ = 13.0267(3)A,V = 3004.70(13)一种3和Ž = 12在NH之间150 K.反应4 F和过量的SbF的5在无水HF导致β - NH 4 Sb 2 F 11的单晶生长:a = 20.0406(7)Å,b = 11.5534(4)Å,c = 13.0796(4)Å,V = 3028.42(18)Å 3, 在296 K时Z =12。在NH 4 Sb 2 F 11上的XRD实验在240和150 K时揭示了另一种修饰(α- NH 4 Sb 2 F 11),其在一个(伪六边形)单斜晶胞空间群P2 1中结晶,a = 11.5045(6)Å,b = 13.0437(3)埃,ç = 11.5264(6)A,β = 119.818(7)°,V = 1500.67(15)一种3,和ž = 6在将O之间240 K.反应2的SbF 6和的SbF 5在无水HF,得到ö 2的Sb 2 ˚F 11在与结晶一 = 19.8472(5),b = 11.1172(3)埃,c ^ = 12.8259(3)A,V = 2829.97(12)一种3,和ž = 12,在150 K的ASB的晶体结构2 ˚F 11 A( = RB +,铊+,O- 2 +,NH 4 +)盐包括二聚的Sb 2 ˚F 11 -具有部分无序终端氟原子的阴离子。阴离子的负电荷由单电荷阳离子补偿。α- NH 4 Sb 2 F 11的晶体结构表现出与β相相似的基序,但N-H…F氢键稍微重排。在100-296 K范围内,仅观察到NH 4 SbF 6的菱面体相(菱面体空间群R 148号)与一个= b = 7.5961(9)埃,c ^ = 7.7176(9)埃,α = β = 90°,γ = 120°,V = 385.65(12)一种3,和ž = 3在150 K)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号