当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of a well-defined polyelectrolyte by controlled/“living” nitroxide-mediated radical polymerization. Kinetic study
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.eurpolymj.2020.109815 Christos Pantazidis , Stelios Andreou , Emmanouil Glynos , Georgios Sakellariou
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.eurpolymj.2020.109815 Christos Pantazidis , Stelios Andreou , Emmanouil Glynos , Georgios Sakellariou
|
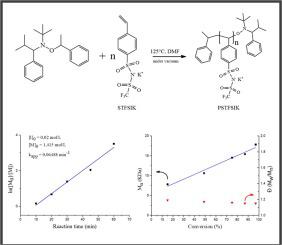
Abstract The synthesis and characterization of potassium 4-styrenesulfonyl (trifluoromethylsulfonyl) imide (STFSIK) monomer as well as its controlled/living nitroxide-mediated radical polymerization (NMRP), using N-tert-Butyl-N-(2-methyl-1-phenylpropyl)-O-(1-phenylethyl) hydroxylamine as initiator in 50% w/v solutions of DMF at 125 °C, are reported. The kinetic studies of STFSIK indicated controlled/“living” polymerization process free of coupling and transfer reactions. 1H NMR and SEC were employed to determine the rate of monomer consumption and molecular weight distribution, Ð, of the synthesized samples. The polymerization reaction established high monomer conversion in just an hour with high end-group fidelity and the ability to continue polymerization with no visible terminations. Finally, the synthesis of the corresponding PSTFSILi homopolymer was achieved through a metathesis reaction between PSTFSIK and LiClO4, and the subsequent purification was successful against dialysis in water.
中文翻译:

通过受控/“活性”氮氧化合物介导的自由基聚合合成定义明确的聚电解质。动力学研究
摘要 使用 N-叔丁基-N-(2-甲基-1-) 合成和表征 4-苯乙烯磺酰基 (三氟甲基磺酰基) 亚胺 (STFSIK) 单体及其受控/活性硝基氧介导的自由基聚合 (NMRP)苯丙基)-O-(1-苯乙基) 羟胺作为引发剂在 125 °C 的 50% w/v DMF 溶液中被报道。STFSIK 的动力学研究表明受控/“活性”聚合过程没有偶联和转移反应。1H NMR 和 SEC 用于确定合成样品的单体消耗率和分子量分布 Ð。聚合反应在短短一个小时内建立了高单体转化率,具有高端基保真度和继续聚合的能力,没有可见的终止。最后,
更新日期:2020-07-01
中文翻译:

通过受控/“活性”氮氧化合物介导的自由基聚合合成定义明确的聚电解质。动力学研究
摘要 使用 N-叔丁基-N-(2-甲基-1-) 合成和表征 4-苯乙烯磺酰基 (三氟甲基磺酰基) 亚胺 (STFSIK) 单体及其受控/活性硝基氧介导的自由基聚合 (NMRP)苯丙基)-O-(1-苯乙基) 羟胺作为引发剂在 125 °C 的 50% w/v DMF 溶液中被报道。STFSIK 的动力学研究表明受控/“活性”聚合过程没有偶联和转移反应。1H NMR 和 SEC 用于确定合成样品的单体消耗率和分子量分布 Ð。聚合反应在短短一个小时内建立了高单体转化率,具有高端基保真度和继续聚合的能力,没有可见的终止。最后,











































 京公网安备 11010802027423号
京公网安备 11010802027423号