Electrochemistry Communications ( IF 4.7 ) Pub Date : 2020-06-05 , DOI: 10.1016/j.elecom.2020.106764 Seongjae Ko , Yuki Yamada , Atsuo Yamada
|
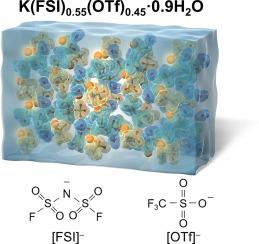
Concentrated aqueous electrolytes have been widely explored for high-voltage aqueous batteries. To achieve higher voltage, even higher alkali-ion molalities are being pursuit up to 55.5 mol kg−1 (m) that corresponds to monohydrate, but the sacrificially lowered ionic conductivity is problematic. Here we report a 61.7 m K-ion aqueous electrolyte composed of KN(SO2F)2 (KFSI) and KSO3CF3 (KOTf). The pioneered beyond-monohydrate realm is unique to K-ion systems, in which [FSI]− anion is stable to hydrolysis. The K-ion electrolyte has two-order higher ionic conductivity (12 mS cm−1 at 30 °C) than a comparable 55.5 m Li-ion system (0.1 mS cm−1) while exhibiting a 2.7 V potential window on Pt being among the widest in all alkali-ion aqueous electrolytes. This work suggests the superior overall performance of aqueous K-ion batteries over other alkali-ion batteries.
中文翻译:

62 m K离子水性电解质
浓缩水性电解质已被广泛地用于高压水性电池。为了获得更高的电压,甚至追求更高的碱金属离子摩尔数,达到相当于一水合物的55.5 mol kg -1(m),但是牺牲地降低离子电导率是有问题的。在这里,我们报告了由KN(SO 2 F)2(KFSI)和KSO 3 CF 3(KOTf)组成的61.7 m K离子水性电解质。开创性的超越一水合物领域是K离子系统独有的,其中[FSI] -阴离子对水解稳定。与可比的55.5 m锂离子系统(0.1 mS cm -1)相比,K离子电解质的离子电导率(30°C下为12 mS cm -1)高两倍。),而在所有碱离子水性电解质中,Pt上的2.7 V电位窗口最大。这项工作表明水性K离子电池的整体性能优于其他碱离子电池。











































 京公网安备 11010802027423号
京公网安备 11010802027423号