Chemical Physics ( IF 2.0 ) Pub Date : 2020-06-04 , DOI: 10.1016/j.chemphys.2020.110860 Yue Zhang , Wenfeng Hu , Jingyue Sun , Yanghui Li , Cong Chen
|
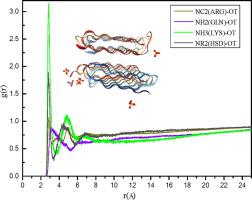
Inhibition of gas hydrate is extremely important in gas hydrate recovery and oil/gas transportation. Antifreeze proteins are environment friendly inhibitors and as one of the most active AFPs up to now, the inhibition mechanism of RiAFP was open to questions. Using molecular dynamics simulations, strong interaction between specific residues of AFPs and water molecules has been found. Residues ASP and ARG have strong ability to form hydrogen bonds with water and strong hydrogen bonding interaction with water. For residues THR, SER and ALA, they also have strong hydrogen bonding interaction with water and the total numbers of hydrogen bonds formed with water are rather large considering the large numbers of these residues in RiAFP. NH2 group in GLN, methyl group in MET and ILE show strong hydration with water. These residues play important role in RiAFP inhibition of ice and gas hydrate either by hydrogen bonding interaction or hydration.
中文翻译:

分子动力学模拟揭示RiAFP与水之间的氢键和水合物相互作用
天然气水合物的抑制在天然气水合物的回收和石油/天然气的运输中极为重要。抗冻蛋白是环境友好的抑制剂,并且作为迄今为止最活跃的AFP之一,RiAFP的抑制机制尚有待商questions。使用分子动力学模拟,已发现AFP的特定残基与水分子之间的强相互作用。残基ASP和ARG具有与水形成氢键的强大能力以及与水的强氢键相互作用。对于残基THR,SER和ALA,它们与水也具有很强的氢键相互作用,考虑到RiAFP中的大量残基,与水形成的氢键总数相当大。NH 2GLN中的基团,MET和ILE中的甲基与水形成强水合。这些残基通过氢键相互作用或水合作用在RiAFP抑制冰和天然气水合物中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号