Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-06-05 , DOI: 10.1016/j.bmc.2020.115578 Anupam Karki 1 , Hari K Namballa 2 , Ian Alberts 3 , Wayne W Harding 4
|
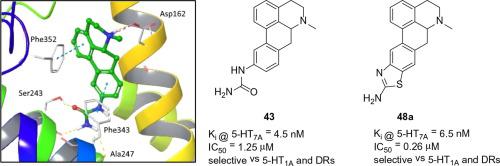
Aporphine alkaloids containing a C10 nitrogen motif were synthesized and evaluated for affinity at 5-HT1AR, 5-HT2AR, 5-HT6R and 5-HT7AR. Three series of racemic aporphines were investigated: 1,2,10-trisubstituted, C10 N-monosubstituted and compounds containing a C10 benzofused aminothiazole moiety. The 1,2,10-trisubstituted series of compounds as a group displayed modest selectivity for 5-HT7AR and also had moderate 5-HT7AR affinity. Compounds from the C10 N-monosubstituted series generally lacked affinity for 5-HT2AR and 5-HT6R and showed strong affinity for 5-HT1A or 5-HT7AR. Compounds in this series that contained an N6-methyl group were up to 27-fold selective for 5-HT7AR over 5-HT1AR, whereas compounds with an N6-propyl substituent showed a reversal in this selectivity. The C10 benzofused aminothiazole analogues showed a similar binding profile as the C10 N-monosubstituted series i.e. strong affinity for 5-HT1AR or 5-HT7AR, with selectivity between the two receptors being similarly influenced by N6-methyl or N6-propyl substituents. Compounds 29 and 34a exhibit high 5-HT7AR affinity, excellent selectivity versus dopamine receptors and function as antagonists in 5-HT7AR cAMP-based assays. Compounds 29 and 34a have been identified as new lead molecules for further tool and pharmaceutical optimization.
中文翻译:

通过 C10 氮化对阿朴啡进行结构操作,从而鉴定出新的 5-HT7AR 配体。
合成了含有 C10 氮基序的阿朴啡生物碱,并评估了对 5-HT 1A R、5-HT 2A R、5-HT 6 R 和 5-HT 7A R 的亲和力。研究了三个系列的外消旋阿朴啡:1,2、 10-三取代、C10 N-单取代和含有 C10 苯并稠合氨基噻唑部分的化合物。1,2,10-三取代系列化合物作为一个组显示出对5-HT 7A R 的适度选择性,并且还具有中等的5-HT 7A R 亲和力。来自 C10 N-单取代系列的化合物通常对 5-HT 2A R 和 5-HT 6 R缺乏亲和力,而对 5-HT 1A表现出很强的亲和力或 5-HT 7A R。该系列中含有N 6-甲基的化合物对 5-HT 7A R 的选择性是5-HT 1A R 的27 倍,而具有N 6-丙基取代基的化合物显示出这种选择性的逆转。C10 苯并稠合氨基噻唑类似物显示出与 C10 N-单取代系列相似的结合特性,即对 5-HT 1AR或 5-HT 7AR具有强亲和力,两种受体之间的选择性同样受N 6-甲基或N 6 的影响-丙基取代基。化合物29和34a表现出高 5-HT 7AR 亲和力、对多巴胺受体的出色选择性,并在基于 5-HT 7A R cAMP 的测定中用作拮抗剂。化合物29和34a已被鉴定为用于进一步工具和药物优化的新先导分子。









































 京公网安备 11010802027423号
京公网安备 11010802027423号