当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into Lewis base-catalyzed chemoselective [3 + 2] and [3 + 4] annulation reactions of MBH carbonates
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-06-04 , DOI: 10.1039/d0qo00457j Qianqian Deng 1, 2, 3, 4, 5 , Shi-Jun Li 1, 2, 3, 4, 5 , Donghui Wei 1, 2, 3, 4, 5 , Yu Lan 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-06-04 , DOI: 10.1039/d0qo00457j Qianqian Deng 1, 2, 3, 4, 5 , Shi-Jun Li 1, 2, 3, 4, 5 , Donghui Wei 1, 2, 3, 4, 5 , Yu Lan 1, 2, 3, 4, 5
Affiliation
|
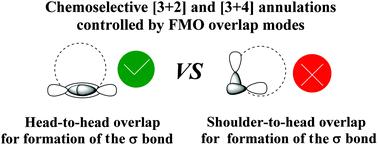
Predicting the chemoselectivity of Lewis base-catalyzed annulations of Morita–Baylis–Hillman (MBH) carbonates is one of the most challenging questions in the organocatalysis field. The origin of chemoselectivity in Lewis base-catalyzed [3 + 4] and [3 + 2] annulations of MBH carbonates was theoretically explored in this work. The calculated results indicate that the general reaction pathway contains five steps: (1) nucleophilic addition on the α-carbon of MBH carbonates by a Lewis base, (2) dissociation of BocO−, (3) deprotonation of the α-carbon, (4) Michael addition, and (5) ring closure coupled with the dissociation of the Lewis base. The ring-closure step determines the chemoselectivity for the possible [3 + 4] and [3 + 2] annulation reactions. The chemoselectivity can be well predicted using frontier molecular orbital (FMO) analyses, which demonstrate that the different overlap modes of FMOs involved in ring-closure transition states control the chemoselectivity.
中文翻译:

刘易斯碱催化MBH碳酸盐的化学选择性[3 + 2]和[3 + 4]环化反应的见解
预测刘易斯碱催化的森田-贝利斯-希尔曼(MBH)碳酸盐的化学选择性是有机催化领域最具挑战性的问题之一。在这项工作中,从理论上探讨了路易斯碱催化的MBH碳酸酯的[3 + 4]和[3 + 2]环中的化学选择性的起源。计算结果表明,一般的反应途径包含五个步骤:(1)在MBH碳酸酯的α碳的亲核加成由路易斯碱,(2)BOCO的解离-,(3)α-碳的去质子化,(4)迈克尔加成,和(5)闭环与路易斯碱的解离相结合。闭环步骤确定了可能的[3 + 4]和[3 + 2]环化反应的化学选择性。使用前沿分子轨道(FMO)分析可以很好地预测化学选择性,这表明参与闭环过渡态的FMO的不同重叠模式控制着化学选择性。
更新日期:2020-07-14
中文翻译:

刘易斯碱催化MBH碳酸盐的化学选择性[3 + 2]和[3 + 4]环化反应的见解
预测刘易斯碱催化的森田-贝利斯-希尔曼(MBH)碳酸盐的化学选择性是有机催化领域最具挑战性的问题之一。在这项工作中,从理论上探讨了路易斯碱催化的MBH碳酸酯的[3 + 4]和[3 + 2]环中的化学选择性的起源。计算结果表明,一般的反应途径包含五个步骤:(1)在MBH碳酸酯的α碳的亲核加成由路易斯碱,(2)BOCO的解离-,(3)α-碳的去质子化,(4)迈克尔加成,和(5)闭环与路易斯碱的解离相结合。闭环步骤确定了可能的[3 + 4]和[3 + 2]环化反应的化学选择性。使用前沿分子轨道(FMO)分析可以很好地预测化学选择性,这表明参与闭环过渡态的FMO的不同重叠模式控制着化学选择性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号