当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of an eleganine A core.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-04 , DOI: 10.1039/d0ob00939c Gints Smits 1 , Ronalds Zemribo 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-04 , DOI: 10.1039/d0ob00939c Gints Smits 1 , Ronalds Zemribo 1
Affiliation
|
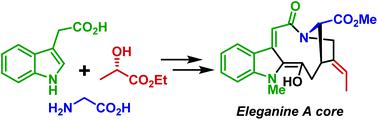
A synthetic approach towards the core of a structurally unique cytotoxic indole alkaloid eleganine A has been accomplished for the first time. The synthesis features a stereoselective Ireland–Claisen rearrangement as the key step, enabling the installation of 2 stereogenic centers and a stereodefined double bond in a single step. Furthermore, a SnCl4 promoted acylation of the indole C-2 position allows the coupling of a highly functionalized 4-ethylidene proline fragment with the indole part.
中文翻译:

eleganine A核心的立体选择性合成。
首次完成了针对结构独特的细胞毒性吲哚生物碱线虫氨酸A核心的合成方法。该合成的关键步骤是将爱尔兰-克莱森立体选择性重排作为关键步骤,从而使一步即可安装2个立体异构中心和一个立体定义的双键。此外,SnCl 4促进的吲哚C-2位置的酰化作用使高度官能化的4-亚乙基脯氨酸片段与吲哚部分偶联。
更新日期:2020-06-24
中文翻译:

eleganine A核心的立体选择性合成。
首次完成了针对结构独特的细胞毒性吲哚生物碱线虫氨酸A核心的合成方法。该合成的关键步骤是将爱尔兰-克莱森立体选择性重排作为关键步骤,从而使一步即可安装2个立体异构中心和一个立体定义的双键。此外,SnCl 4促进的吲哚C-2位置的酰化作用使高度官能化的4-亚乙基脯氨酸片段与吲哚部分偶联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号