当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of cycloiptycenes from carbon nanobelts.
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-04 , DOI: 10.1039/d0sc02501a Hiroki Shudo 1 , Motonobu Kuwayama 2, 3 , Yasutomo Segawa 1, 2, 4, 5 , Kenichiro Itami 1, 2, 3
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-04 , DOI: 10.1039/d0sc02501a Hiroki Shudo 1 , Motonobu Kuwayama 2, 3 , Yasutomo Segawa 1, 2, 4, 5 , Kenichiro Itami 1, 2, 3
Affiliation
|
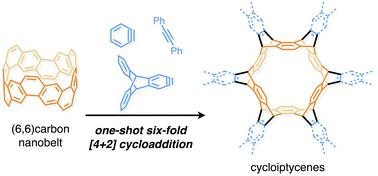
The synthesis of each of the cycloiptycene derivatives was achieved in one step from the (6,6)carbon nanobelt. It was revealed that the carbon nanobelt reacted as a diene in the Diels–Alder reaction with arynes and alkynes. The structures of all products were identified by X-ray crystallography to confirm that the Diels–Alder reactions took place at the six central benzene rings of the carbon nanobelt. DFT calculations indicated that the release of strain energy is the driving force to promote the Diels–Alder reaction. By using this method, we have successfully synthesized cyclotetracosiptycene, the largest iptycene ever synthesized.
中文翻译:

从碳纳米带合成环烯。
每种环烯衍生物的合成都是由(6,6)碳纳米带一步完成的。研究表明,碳纳米带在狄尔斯-阿尔德反应中与芳烃和炔烃发生反应,形成二烯。所有产物的结构均通过X射线晶体学鉴定,证实Diels-Alder反应发生在碳纳米带的六个中心苯环上。DFT计算表明应变能的释放是促进Diels-Alder反应的驱动力。通过该方法,我们成功合成了环四环四烯,这是迄今为止合成的最大的环四烯。
更新日期:2020-07-08
中文翻译:

从碳纳米带合成环烯。
每种环烯衍生物的合成都是由(6,6)碳纳米带一步完成的。研究表明,碳纳米带在狄尔斯-阿尔德反应中与芳烃和炔烃发生反应,形成二烯。所有产物的结构均通过X射线晶体学鉴定,证实Diels-Alder反应发生在碳纳米带的六个中心苯环上。DFT计算表明应变能的释放是促进Diels-Alder反应的驱动力。通过该方法,我们成功合成了环四环四烯,这是迄今为止合成的最大的环四烯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号