当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Mechanism of Thiol-Triggered COS/H2S Release from N-Dithiasuccinoyl Amines.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.joc.0c00559 Shengchao Zhou 1 , Yujie Mou 2 , Miao Liu 1 , Qian Du 2 , Basharat Ali 1 , Jurupula Ramprasad 1 , Chunhua Qiao 1 , Li-Fang Hu 2, 3 , Xingyue Ji 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.joc.0c00559 Shengchao Zhou 1 , Yujie Mou 2 , Miao Liu 1 , Qian Du 2 , Basharat Ali 1 , Jurupula Ramprasad 1 , Chunhua Qiao 1 , Li-Fang Hu 2, 3 , Xingyue Ji 1
Affiliation
|
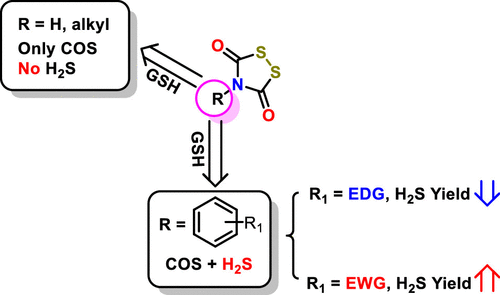
The hydrolysis of carbonyl sulfide (COS) to form H2S by carbonic anhydrase has been demonstrated to be a viable strategy to deliver H2S in a biological system. Herein, we describe N-dithiasuccinoyl amines as thiol-triggered COS/H2S donors. Notably, thiol species especially GSH and homocysteine can trigger the release of both COS and H2S directly from several specific analogues via an unexpected mechanism. Importantly, two representative analogues Dts-1 and Dts-5 show intracellular H2S release, and Dts-1 imparts potent anti-inflammatory effects in LPS-challenged microglia cells. In conclusion, N-dithiasuccinoyl amine could serve as promising COS/H2S donors for either H2S biological studies or H2S-based therapeutics development.
中文翻译:

N-二硫代琥珀酰胺中硫醇引发的COS / H2S释放机理的见解。
碳酸酐酶水解羰基硫(COS)形成H 2 S已被证明是在生物系统中递送H 2 S的可行策略。在本文中,我们将N-二硫代琥珀酰胺描述为硫醇触发的COS / H 2 S供体。值得注意的是,硫醇物种尤其GSH和高半胱氨酸可以触发COS和H的释放2通过一个意想不到的机构S直接从几个特定的类似物。重要的是,两个代表性的类似物Dts-1和Dts-5显示了细胞内H 2 S的释放,而Dts-1在LPS挑战的小胶质细胞中具有强大的抗炎作用。结论,N-二硫代琥珀酰胺可作为有前途的COS / H 2 S供体,用于H 2 S生物学研究或基于H 2 S的治疗方法开发。
更新日期:2020-07-02
中文翻译:

N-二硫代琥珀酰胺中硫醇引发的COS / H2S释放机理的见解。
碳酸酐酶水解羰基硫(COS)形成H 2 S已被证明是在生物系统中递送H 2 S的可行策略。在本文中,我们将N-二硫代琥珀酰胺描述为硫醇触发的COS / H 2 S供体。值得注意的是,硫醇物种尤其GSH和高半胱氨酸可以触发COS和H的释放2通过一个意想不到的机构S直接从几个特定的类似物。重要的是,两个代表性的类似物Dts-1和Dts-5显示了细胞内H 2 S的释放,而Dts-1在LPS挑战的小胶质细胞中具有强大的抗炎作用。结论,N-二硫代琥珀酰胺可作为有前途的COS / H 2 S供体,用于H 2 S生物学研究或基于H 2 S的治疗方法开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号