当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of On-Resin Disulfide Formation for Large-Scale Manufacturing of Cyclic Peptides: A Case Study
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.oprd.0c00168 Yi Yang 1 , Lena Hansen 1 , Fabrizio Badalassi 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.oprd.0c00168 Yi Yang 1 , Lena Hansen 1 , Fabrizio Badalassi 1
Affiliation
|
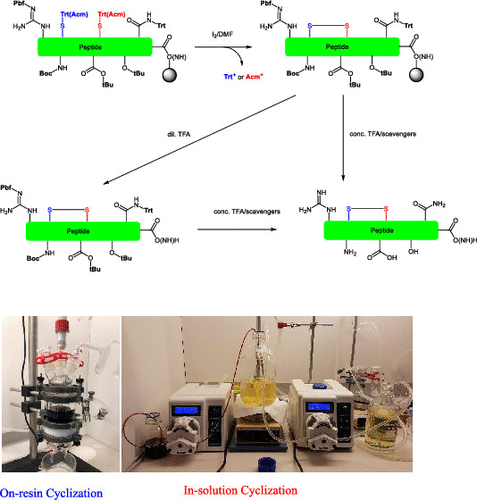
Peptide molecules bearing disulfide moieties constitute an important category of therapeutics. The establishment of the disulfide structure plays a pivotal role in the production of those peptides. In-solution cyclization is generally adopted in industrial peptide production as a synthetic strategy to build disulfide from the linear deprotected precursor. This methodology is inherently limited by the relatively low productivity, considering the minimizing intermolecular oligomerization by dilution of the overall concentration of the reaction. Alternatively, the disulfide bond could be established from the thiol-protected building blocks directly on the solid support. On-resin disulfide construction was pursued for atosiban process development. Protected linear atosiban resin was treated with I2/dimethylformamide (DMF), and the target disulfide was built rapidly and quantitatively within 30 min. The effects of the resin linker and thiol-protecting groups were tested by utilizing Sieber resin, Rink amide resin, and Ramage resin as solid supports and Cys(Trt)/Cys(Acm) as building blocks. The I2 equivalence, as a potential critical process parameter, was subjected to a proven acceptable range (PAR) study to facilitate the envisioned large-scale manufacturing. Although there was rapid and complete disulfide formation on the solid support in all tested combinations of resin and thiol-protecting groups, a reduction of the disulfide was observed in the processes of a certain peptidyl resin cleavage/global deprotection. A dedicated investigation revealed that hydrosilane, employed as a cation scavenger in peptide synthesis, is responsible for the disulfide degradation in the presence of trifluoroacetic acid (TFA). Eliminating silane from the TFA cleavage solution or subtly controlling its equivalence could effectively suppress the nocuous disulfide reduction in this process. In summary, on-resin disulfide cyclization has been verified to be a viable strategy for atosiban industrial production by this study.
中文翻译:

大规模生产环肽的树脂上二硫化物形成的研究:一个案例研究
带有二硫键部分的肽分子构成治疗剂的重要类别。二硫键结构的建立在那些肽的产生中起关键作用。在工业肽生产中通常采用溶液环化作为从线性脱保护的前体生成二硫键的合成策略。考虑到通过稀释反应的总浓度来最小化分子间低聚,这种方法固有地受到相对较低生产率的限制。或者,可以从直接在固体载体上的巯基保护的结构单元建立二硫键。在阿托西班工艺开发中追求树脂上二硫化物的建设。受保护的线性阿托西班树脂用I 2处理/二甲基甲酰胺(DMF),并在30分钟内快速定量建立了目标二硫键。通过使用Sieber树脂,Rink酰胺树脂和Ramage树脂作为固体载体,并以Cys(Trt)/ Cys(Acm)作为结构单元,测试了树脂接头和硫醇保护基的作用。我2等效性作为潜在的关键工艺参数,经过了公认的可接受范围(PAR)研究,以促进设想的大规模生产。尽管在所有测试的树脂和硫醇保护基组合中在固体载体上均快速而完全地形成了二硫化物,但在某些肽基树脂裂解/整体脱保护过程中观察到二硫化物的减少。一项专门的研究表明,在肽合成中用作阳离子清除剂的氢硅烷在三氟乙酸(TFA)存在下是导致二硫化物降解的原因。从TFA裂解溶液中除去硅烷或巧妙地控制其当量可以有效地抑制此过程中有害的二硫化物还原。综上所述,
更新日期:2020-07-17
中文翻译:

大规模生产环肽的树脂上二硫化物形成的研究:一个案例研究
带有二硫键部分的肽分子构成治疗剂的重要类别。二硫键结构的建立在那些肽的产生中起关键作用。在工业肽生产中通常采用溶液环化作为从线性脱保护的前体生成二硫键的合成策略。考虑到通过稀释反应的总浓度来最小化分子间低聚,这种方法固有地受到相对较低生产率的限制。或者,可以从直接在固体载体上的巯基保护的结构单元建立二硫键。在阿托西班工艺开发中追求树脂上二硫化物的建设。受保护的线性阿托西班树脂用I 2处理/二甲基甲酰胺(DMF),并在30分钟内快速定量建立了目标二硫键。通过使用Sieber树脂,Rink酰胺树脂和Ramage树脂作为固体载体,并以Cys(Trt)/ Cys(Acm)作为结构单元,测试了树脂接头和硫醇保护基的作用。我2等效性作为潜在的关键工艺参数,经过了公认的可接受范围(PAR)研究,以促进设想的大规模生产。尽管在所有测试的树脂和硫醇保护基组合中在固体载体上均快速而完全地形成了二硫化物,但在某些肽基树脂裂解/整体脱保护过程中观察到二硫化物的减少。一项专门的研究表明,在肽合成中用作阳离子清除剂的氢硅烷在三氟乙酸(TFA)存在下是导致二硫化物降解的原因。从TFA裂解溶液中除去硅烷或巧妙地控制其当量可以有效地抑制此过程中有害的二硫化物还原。综上所述,











































 京公网安备 11010802027423号
京公网安备 11010802027423号