当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined Intranasal Nanoemulsion and RIG-I Activating RNA Adjuvants Enhance Mucosal, Humoral, and Cellular Immunity to Influenza Virus
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.molpharmaceut.0c00315 Pamela T Wong 1, 2, 3 , Peter H Goff 4, 5 , Rachel J Sun 1, 2 , Matthew J Ruge 1, 2 , Megan E Ermler 4 , Alyssa Sebring 1, 2 , Jessica J O'Konek 1, 2, 3 , Jeffrey J Landers 1, 2, 3 , Katarzyna W Janczak 1, 2, 3 , Weina Sun 4 , James R Baker 1, 2, 3
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.molpharmaceut.0c00315 Pamela T Wong 1, 2, 3 , Peter H Goff 4, 5 , Rachel J Sun 1, 2 , Matthew J Ruge 1, 2 , Megan E Ermler 4 , Alyssa Sebring 1, 2 , Jessica J O'Konek 1, 2, 3 , Jeffrey J Landers 1, 2, 3 , Katarzyna W Janczak 1, 2, 3 , Weina Sun 4 , James R Baker 1, 2, 3
Affiliation
|
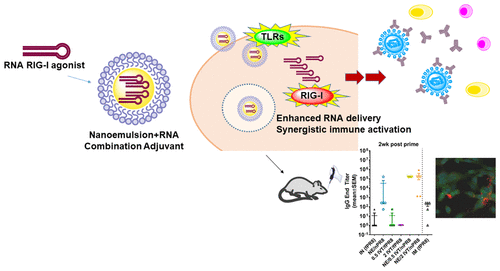
Current influenza virus vaccines are focused on humoral immunity and are limited by the short duration of protection, narrow cross-strain efficacy, and suboptimal immunogenicity. Here, we combined two chemically and biologically distinct adjuvants, an oil-in-water nanoemulsion (NE) and RNA-based agonists of RIG-I, to determine whether the diverse mechanisms of these adjuvants could lead to improved immunogenicity and breadth of protection against the influenza virus. NE activates TLRs, stimulates immunogenic apoptosis, and enhances cellular antigen uptake, leading to a balanced TH1/TH2/TH17 response when administered intranasally. RIG-I agonists included RNAs derived from Sendai and influenza viral defective interfering RNAs (IVT DI, 3php, respectively) and RIG-I/TLR3 agonist, poly(I:C) (pIC), which induce IFN-Is and TH1-polarized responses. NE/RNA combined adjuvants potentially allow for costimulation of multiple innate immune receptor pathways, more closely mimicking patterns of activation occurring during natural viral infection. Mice intranasally immunized with inactivated A/Puerto Rico/8/1934 (H1N1) (PR/8) adjuvanted with NE/IVT DI or NE/3php (but not NE/pIC) showed synergistic enhancement of systemic PR/8-specific IgG with significantly greater avidity and virus neutralization activity than the individual adjuvants. Notably, NE/IVT DI induced protective neutralizing titers after a single immunization. Hemagglutinin stem-specific antibodies were also improved, allowing recognition of heterologous and heterosubtypic hemagglutinins. All NE/RNAs elicited substantial PR/8-specific sIgA. Finally, a unique cellular response with enhanced TH1/TH17 immunity was induced with the NE/RNAs. These results demonstrate that the enhanced immunogenicity of the adjuvant combinations was synergistic and not simply additive, highlighting the potential value of a combined adjuvant approach for improving the efficacy of vaccination against the influenza virus.
中文翻译:

联合鼻内纳米乳剂和 RIG-I 激活 RNA 佐剂增强粘膜、体液和细胞对流感病毒的免疫力
目前的流感病毒疫苗侧重于体液免疫,并且受到保护持续时间短、交叉菌株效力窄和免疫原性欠佳的限制。在这里,我们结合了两种化学和生物学上不同的佐剂,一种水包油纳米乳剂 (NE) 和 RIG-I 的基于 RNA 的激动剂,以确定这些佐剂的不同机制是否可以提高免疫原性和保护范围流感病毒。NE 激活 TLR,刺激免疫原性细胞凋亡,并增强细胞抗原摄取,导致平衡的 T H 1/T H 2/T H17 鼻内给药时的反应。RIG-I 激动剂包括源自仙台的 RNA 和流感病毒缺陷干扰 RNA(分别为 IVT DI,3php)和 RIG-I/TLR3 激动剂 poly(I:C) (pIC),可诱导 IFN-Is 和 T H1-极化反应。NE/RNA 组合佐剂可能允许共刺激多种先天免疫受体途径,更接近地模拟自然病毒感染期间发生的激活模式。用灭活的 A/Puerto Rico/8/1934 (H1N1) (PR/8) 以 NE/IVT DI 或 NE/3php(但不是 NE/pIC)为佐剂的小鼠鼻内免疫显示全身性 PR/8 特异性 IgG 与比单个佐剂显着更高的亲和力和病毒中和活性。值得注意的是,NE/IVT DI 在单次免疫后诱导了保护性中和效价。血凝素干特异性抗体也得到了改进,可以识别异源和异亚型血凝素。所有 NE/RNA 均引发大量 PR/8 特异性 sIgA。最后,具有增强 T 的独特细胞反应H 1/T H 17 免疫是用 NE/RNA 诱导的。这些结果表明,佐剂组合增强的免疫原性是协同的,而不是简单的相加,突出了组合佐剂方法在提高针对流感病毒的疫苗接种效力方面的潜在价值。
更新日期:2020-06-03
中文翻译:

联合鼻内纳米乳剂和 RIG-I 激活 RNA 佐剂增强粘膜、体液和细胞对流感病毒的免疫力
目前的流感病毒疫苗侧重于体液免疫,并且受到保护持续时间短、交叉菌株效力窄和免疫原性欠佳的限制。在这里,我们结合了两种化学和生物学上不同的佐剂,一种水包油纳米乳剂 (NE) 和 RIG-I 的基于 RNA 的激动剂,以确定这些佐剂的不同机制是否可以提高免疫原性和保护范围流感病毒。NE 激活 TLR,刺激免疫原性细胞凋亡,并增强细胞抗原摄取,导致平衡的 T H 1/T H 2/T H17 鼻内给药时的反应。RIG-I 激动剂包括源自仙台的 RNA 和流感病毒缺陷干扰 RNA(分别为 IVT DI,3php)和 RIG-I/TLR3 激动剂 poly(I:C) (pIC),可诱导 IFN-Is 和 T H1-极化反应。NE/RNA 组合佐剂可能允许共刺激多种先天免疫受体途径,更接近地模拟自然病毒感染期间发生的激活模式。用灭活的 A/Puerto Rico/8/1934 (H1N1) (PR/8) 以 NE/IVT DI 或 NE/3php(但不是 NE/pIC)为佐剂的小鼠鼻内免疫显示全身性 PR/8 特异性 IgG 与比单个佐剂显着更高的亲和力和病毒中和活性。值得注意的是,NE/IVT DI 在单次免疫后诱导了保护性中和效价。血凝素干特异性抗体也得到了改进,可以识别异源和异亚型血凝素。所有 NE/RNA 均引发大量 PR/8 特异性 sIgA。最后,具有增强 T 的独特细胞反应H 1/T H 17 免疫是用 NE/RNA 诱导的。这些结果表明,佐剂组合增强的免疫原性是协同的,而不是简单的相加,突出了组合佐剂方法在提高针对流感病毒的疫苗接种效力方面的潜在价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号