当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modeling the Solution Properties and Mineral–Solution Equilibria in Radionuclide-Bearing Aqueous Nitrate Systems: Application to Binary and Ternary Systems Containing U, Th, or Lanthanides at 25 °C
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.jced.0c00180 Arnault Lassin 1 , Sylvain Guignot 1 , Adeline Lach 1 , Christomir Christov 2 , Laurent André 1, 3 , Benoît Madé 4
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.jced.0c00180 Arnault Lassin 1 , Sylvain Guignot 1 , Adeline Lach 1 , Christomir Christov 2 , Laurent André 1, 3 , Benoît Madé 4
Affiliation
|
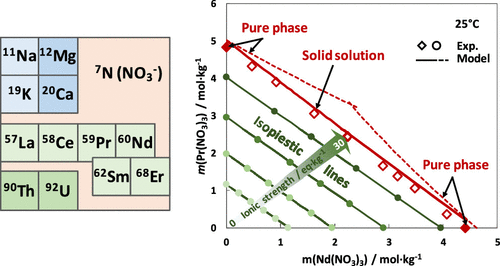
This article focuses on the modeling of the thermodynamic properties of aqueous nitrate systems that contain radionuclides from low molalities to saturation and occasionally supersaturation with respect to the corresponding nitrate solid salts. It is an additional contribution following previous works dedicated to nitrate systems containing alkali and/or alkali-earth metals or lanthanides. Here, 18 chemical systems, mostly ternary, were studied at 25 °C: 5 contained actinides (Th or U(IV)) and 13 contained lanthanum and/or lanthanides (Ce, Pr, Nd, Sm, and/or Er). Six of these systems also contained alkali (Na, K) or alkali-earth metals (Mg, Ca). The modeling approach was based on the standard Pitzer formulation for strong aqueous electrolytes and was used to reproduce the published experimental data on the osmotic coefficient of solutions and on the solubility diagrams of salts. Ion-specific ternary interaction parameters and nitrate salt solubility products are proposed. The results suggest that ternary nitrate systems containing two lanthanides with close atomic numbers, such as Pr and Nd or Nd and Sm, are favorable to the formation of solid solutions. Otherwise, pure salts precipitate in their own stability domain. This allows us to propose predictive solubility diagrams for Ln–Er–NO3–H2O and Ln–Ce–NO3–H2O systems (with Ln = La, Pr, or Nd), the former being controlled by pure salts and the latter being controlled by ideal solid solutions.
中文翻译:

在含放射性核素的硝酸水溶液系统中模拟溶液的性质和矿物质溶液的平衡:在25°C下应用于含U,Th或镧系元素的二元和三元系统
本文着重于硝酸盐水溶液系统的热力学特性建模,该系统包含相对于相应的硝酸盐固体盐而言从低摩尔浓度到饱和度(有时是过饱和度)的放射性核素。这是在先前致力于含碱和/或碱土金属或镧系元素的硝酸盐体系的工作之后的又一贡献。在这里,在25°C下研究了18种化学体系,大部分是三元体系:5种化学系act系元素(Th或U(IV)),13种化学系镧和/或镧系元素(Ce,Pr,Nd,Sm和/或Er)。这些系统中的六个还包含碱金属(Na,K)或碱土金属(Mg,Ca)。该建模方法基于用于强水电解质的标准Pitzer配方,并用于复制已发布的有关溶液的渗透系数和盐的溶解度图的实验数据。提出了离子特异性三元相互作用参数和硝酸盐溶解度产物。结果表明,含有两个原子序数接近的镧系元素的三元硝酸盐体系(例如Pr和Nd或Nd和Sm)有利于固溶体的形成。否则,纯盐会在其自身的稳定性域中沉淀。这使我们能够提出Ln–Er–NO的预测溶解度图 结果表明,含有两个原子序数接近的镧系元素的三元硝酸盐体系(例如Pr和Nd或Nd和Sm)有利于固溶体的形成。否则,纯盐会在其自身的稳定性域中沉淀。这使我们能够提出Ln–Er–NO的预测溶解度图 结果表明,含有两个原子序数接近的镧系元素的三元硝酸盐体系(例如Pr和Nd或Nd和Sm)有利于固溶体的形成。否则,纯盐会在其自身的稳定性域中沉淀。这使我们能够提出Ln–Er–NO的预测溶解度图3 –H 2 O和Ln–Ce–NO 3 –H 2 O系统(Ln = La,Pr或Nd),前者由纯盐控制,后者由理想固溶体控制。
更新日期:2020-07-09
中文翻译:

在含放射性核素的硝酸水溶液系统中模拟溶液的性质和矿物质溶液的平衡:在25°C下应用于含U,Th或镧系元素的二元和三元系统
本文着重于硝酸盐水溶液系统的热力学特性建模,该系统包含相对于相应的硝酸盐固体盐而言从低摩尔浓度到饱和度(有时是过饱和度)的放射性核素。这是在先前致力于含碱和/或碱土金属或镧系元素的硝酸盐体系的工作之后的又一贡献。在这里,在25°C下研究了18种化学体系,大部分是三元体系:5种化学系act系元素(Th或U(IV)),13种化学系镧和/或镧系元素(Ce,Pr,Nd,Sm和/或Er)。这些系统中的六个还包含碱金属(Na,K)或碱土金属(Mg,Ca)。该建模方法基于用于强水电解质的标准Pitzer配方,并用于复制已发布的有关溶液的渗透系数和盐的溶解度图的实验数据。提出了离子特异性三元相互作用参数和硝酸盐溶解度产物。结果表明,含有两个原子序数接近的镧系元素的三元硝酸盐体系(例如Pr和Nd或Nd和Sm)有利于固溶体的形成。否则,纯盐会在其自身的稳定性域中沉淀。这使我们能够提出Ln–Er–NO的预测溶解度图 结果表明,含有两个原子序数接近的镧系元素的三元硝酸盐体系(例如Pr和Nd或Nd和Sm)有利于固溶体的形成。否则,纯盐会在其自身的稳定性域中沉淀。这使我们能够提出Ln–Er–NO的预测溶解度图 结果表明,含有两个原子序数接近的镧系元素的三元硝酸盐体系(例如Pr和Nd或Nd和Sm)有利于固溶体的形成。否则,纯盐会在其自身的稳定性域中沉淀。这使我们能够提出Ln–Er–NO的预测溶解度图3 –H 2 O和Ln–Ce–NO 3 –H 2 O系统(Ln = La,Pr或Nd),前者由纯盐控制,后者由理想固溶体控制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号