当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calorimetric Determination of Cocrystal Thermodynamic Stability: Sulfamethazine–Salicylic Acid Case Study
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.cgd.9b01253 Michael Svärd 1, 2 , Dipali Ahuja 2 , Åke C. Rasmuson 1, 2
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.cgd.9b01253 Michael Svärd 1, 2 , Dipali Ahuja 2 , Åke C. Rasmuson 1, 2
Affiliation
|
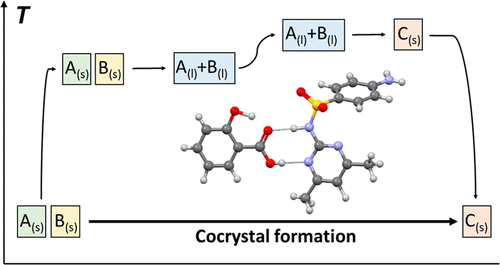
This work demonstrates how the thermodynamics of cocrystal formation from the pure, solid coformers can be directly determined from experimentally obtained calorimetric data, without involving solubility data or approximations of ideal solution. For the 1:1 cocrystal between the drug API sulfamethazine and salicylic acid, the melting temperatures and associated enthalpies of fusion have been determined for the coformers in their respective pure solid state and as an equimolar physical mixture and for the cocrystal, using differential scanning calorimetry. Heat capacities have been determined for the respective solid forms and their supercooled melts. The Gibbs energy for cocrystal formation and the enthalpic and entropic components have been determined as functions of temperature through a thermodynamic cycle. The Gibbs energy, enthalpy, and entropy of mixing have been estimated from the thermodynamic functions for cocrystal formation and fusion of the solid phases. The results show that the Gibbs energy for cocrystal formation is negative, i.e. the cocrystal is the stable solid phase in relation to a 1:1 mixture of the coformers throughout the temperature interval from room temperature to the cocrystal melting point, and becomes increasingly negative with increasing temperature. Cocrystal formation is an endothermic process, driven by the favorable entropy increase, and is accompanied by a 6% increase in molecular volume. At room temperature, liquid mixing of coformers is found to be weakly exothermic. The results qualitatively align with a previously reported analysis based on solubility data.
中文翻译:

量热法测定共晶热力学稳定性:磺胺二甲嘧啶-水杨酸案例研究
这项工作证明了如何从实验获得的量热数据中直接确定由纯的固体共形成物形成共晶的热力学,而无需涉及溶解度数据或理想溶液的近似值。对于药物API磺胺二甲嘧啶和水杨酸之间的1:1共结晶,已使用差示扫描量热法确定了各自纯净状态,等摩尔物理混合物形式的共形成剂的熔融温度和相关的熔融焓。已经确定了各个固体形式及其过冷熔体的热容量。通过热力学循环,已经确定了用于共晶形成的吉布斯能量以及焓和熵组分是温度的函数。吉布斯能量,焓,从共晶形成和固相熔融的热力学函数估计了混合和混合熵。结果表明,共晶形成的吉布斯能量为负,即在从室温到共晶熔点的整个温度区间内,共晶相对于共沸物的1:1混合物而言是稳定的固相,并随着温度的升高而逐渐减小。温度升高。共晶体的形成是吸热过程,受熵的增加所驱动,并伴随着分子体积增加6%。在室温下,发现共形成剂的液体混合微弱地放热。结果与基于溶解度数据的先前报道的分析定性地吻合。
更新日期:2020-07-01
中文翻译:

量热法测定共晶热力学稳定性:磺胺二甲嘧啶-水杨酸案例研究
这项工作证明了如何从实验获得的量热数据中直接确定由纯的固体共形成物形成共晶的热力学,而无需涉及溶解度数据或理想溶液的近似值。对于药物API磺胺二甲嘧啶和水杨酸之间的1:1共结晶,已使用差示扫描量热法确定了各自纯净状态,等摩尔物理混合物形式的共形成剂的熔融温度和相关的熔融焓。已经确定了各个固体形式及其过冷熔体的热容量。通过热力学循环,已经确定了用于共晶形成的吉布斯能量以及焓和熵组分是温度的函数。吉布斯能量,焓,从共晶形成和固相熔融的热力学函数估计了混合和混合熵。结果表明,共晶形成的吉布斯能量为负,即在从室温到共晶熔点的整个温度区间内,共晶相对于共沸物的1:1混合物而言是稳定的固相,并随着温度的升高而逐渐减小。温度升高。共晶体的形成是吸热过程,受熵的增加所驱动,并伴随着分子体积增加6%。在室温下,发现共形成剂的液体混合微弱地放热。结果与基于溶解度数据的先前报道的分析定性地吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号