当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Strong Relationships between Ligand Conformers and Activation Barriers: A Case Study of Bisphosphine Reductive Elimination
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-03 , DOI: 10.1021/acscatal.0c00618 Andrew K. Vitek 1 , Timothy M. E. Jugovic 1 , Paul M. Zimmerman 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-03 , DOI: 10.1021/acscatal.0c00618 Andrew K. Vitek 1 , Timothy M. E. Jugovic 1 , Paul M. Zimmerman 1
Affiliation
|
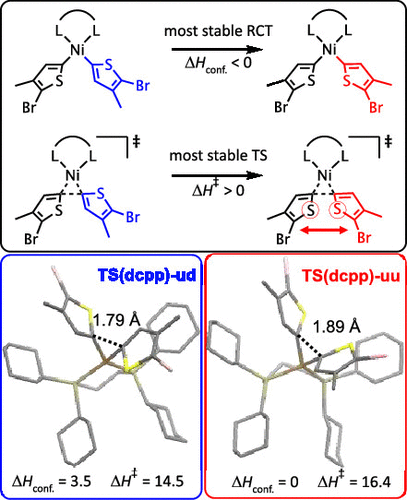
Quantum chemical models of reaction pathways can provide deep insight into the inner workings of transition metal complexes. Often, these simulations have relied on atomistic models where a single or a few conformational isomers of the complex are investigated. This Article will show that, for bisphosphine Ni complexes used to forge C–C bonds, a large number of conformers must be studied to provide confidence that the overall model is meaningful. Not only do conformer effects modify particular reaction barriers, but often the lowest barrier reaction pathway proceeds from a conformer that is not the lowest energy conformer. This finding suggests that errors on the order of more than a few kcal/mol could be present in single-conformer studies. The particular reaction pathway and conformer preferences for a series of eight common Ni bisphosphine complexes will provide some guidance as to when the effects of the conformer will be large or small.
中文翻译:

揭示配体构象异构体与激活障碍之间的紧密关系:以双膦还原消除为例
反应途径的量子化学模型可以深入了解过渡金属配合物的内部工作原理。通常,这些模拟依赖于原子模型,其中研究了复合物的单个或几个构象异构体。本文将表明,对于用于锻造C–C键的双膦Ni配合物,必须研究大量构象异构体,以使人们确信整体模型是有意义的。构象效应不仅会修饰特定的反应障碍,而且最低的障碍反应途径通常来自不是最低能量构象的构象。这一发现表明,在单一致性研究中可能存在超过几千卡/摩尔的误差。
更新日期:2020-07-02
中文翻译:

揭示配体构象异构体与激活障碍之间的紧密关系:以双膦还原消除为例
反应途径的量子化学模型可以深入了解过渡金属配合物的内部工作原理。通常,这些模拟依赖于原子模型,其中研究了复合物的单个或几个构象异构体。本文将表明,对于用于锻造C–C键的双膦Ni配合物,必须研究大量构象异构体,以使人们确信整体模型是有意义的。构象效应不仅会修饰特定的反应障碍,而且最低的障碍反应途径通常来自不是最低能量构象的构象。这一发现表明,在单一致性研究中可能存在超过几千卡/摩尔的误差。











































 京公网安备 11010802027423号
京公网安备 11010802027423号