当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structure of an Nω-hydroxy-L-arginine hydrolase found in the D-cycloserine biosynthetic pathway.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-06-04 , DOI: 10.1107/s2059798320004908 Kosuke Oda 1 , Natsuki Shimotani 2 , Teruo Kuroda 2 , Yasuyuki Matoba 2
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-06-04 , DOI: 10.1107/s2059798320004908 Kosuke Oda 1 , Natsuki Shimotani 2 , Teruo Kuroda 2 , Yasuyuki Matoba 2
Affiliation
|
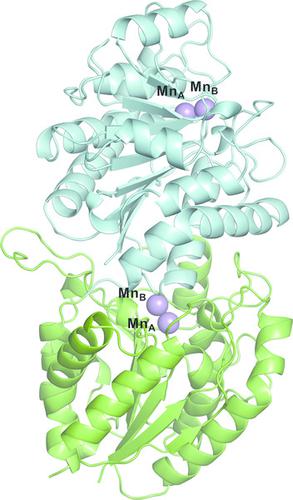
DcsB, one of the enzymes encoded in the d‐cycloserine (d‐CS) biosynthetic gene cluster, displays a high sequence homology to arginase, which contains two manganese ions in the active site. However, DcsB hydrolyzes Nω‐hydroxy‐l‐arginine, but not l‐arginine, to supply hydroxyurea for the biosynthesis of d‐CS. Here, the crystal structure of DcsB was determined at a resolution of 1.5 Å using anomalous scattering from the manganese ions. In the crystal structure, DscB generates an artificial dimer created by the open and closed forms. Gel‐filtration analysis demonstrated that DcsB is a monomeric protein, unlike arginase, which forms a trimeric structure. The active center containing the binuclear manganese cluster differs between DcsB and arginase. In DcsB, one of the ligands of the MnA ion is a cysteine, while the corresponding residue in arginase is a histidine. In addition, DcsB has no counterpart to the histidine residue that acts as a general acid/base during the catalytic reaction of arginase. The present study demonstrates that DcsB has a unique active site that differs from that of arginase.
中文翻译:

在D-环丝氨酸生物合成途径中发现的Nω-羟基-L-精氨酸水解酶的晶体结构。
DcsB是d-环丝氨酸(d- CS)生物合成基因簇中编码的酶之一,与精氨酸酶具有高度的序列同源性,精氨酸酶的活性位点中含有两个锰离子。然而,DcsB水解Ñ ω羟基升精氨酸,但不是升精氨酸,以供应为羟基脲的生物合成d‐CS。此处,使用来自锰离子的异常散射,以1.5的分辨率确定了DcsB的晶体结构。在晶体结构中,DscB生成由开放和封闭形式产生的人工二聚体。凝胶过滤分析表明,DcsB是一种单体蛋白,与精氨酸酶不同,后者形成三聚体结构。DcsB和精氨酸酶的活性中心包含双核锰簇。在DcsB中,Mn A离子的配体之一是半胱氨酸,而精氨酸酶中的相应残基是组氨酸。另外,DcsB与在精氨酸酶催化反应期间充当一般酸/碱的组氨酸残基没有对应物。本研究表明,DcsB具有一个独特的活性位点,不同于精氨酸酶。
更新日期:2020-06-04
中文翻译:

在D-环丝氨酸生物合成途径中发现的Nω-羟基-L-精氨酸水解酶的晶体结构。
DcsB是d-环丝氨酸(d- CS)生物合成基因簇中编码的酶之一,与精氨酸酶具有高度的序列同源性,精氨酸酶的活性位点中含有两个锰离子。然而,DcsB水解Ñ ω羟基升精氨酸,但不是升精氨酸,以供应为羟基脲的生物合成d‐CS。此处,使用来自锰离子的异常散射,以1.5的分辨率确定了DcsB的晶体结构。在晶体结构中,DscB生成由开放和封闭形式产生的人工二聚体。凝胶过滤分析表明,DcsB是一种单体蛋白,与精氨酸酶不同,后者形成三聚体结构。DcsB和精氨酸酶的活性中心包含双核锰簇。在DcsB中,Mn A离子的配体之一是半胱氨酸,而精氨酸酶中的相应残基是组氨酸。另外,DcsB与在精氨酸酶催化反应期间充当一般酸/碱的组氨酸残基没有对应物。本研究表明,DcsB具有一个独特的活性位点,不同于精氨酸酶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号