当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crown-ether-mediated crystal structures of the glycosyltransferase PaGT3 from Phytolacca americana.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-06-04 , DOI: 10.1107/s2059798320005306 Rakesh Maharjan 1 , Yohta Fukuda 1 , Taisuke Nakayama 2 , Toru Nakayama 3 , Hiroki Hamada 4 , Shin Ichi Ozaki 5 , Tsuyoshi Inoue 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-06-04 , DOI: 10.1107/s2059798320005306 Rakesh Maharjan 1 , Yohta Fukuda 1 , Taisuke Nakayama 2 , Toru Nakayama 3 , Hiroki Hamada 4 , Shin Ichi Ozaki 5 , Tsuyoshi Inoue 1
Affiliation
|
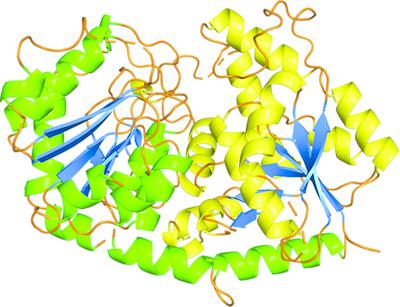
Uridine diphosphate glycosyltransferases (UGTs) are ubiquitous enzymes that are involved in the glycosylation of small molecules. As glycosylation improves the water solubility and stability of hydrophobic compounds, interest in the use of UGTs for the synthesis of glycosides of poorly soluble compounds is increasing. While sugar‐donor recognition in UGTs is conserved with the presence of a plant secondary product glycosyltransferase (PSPG) motif, the basis of the recognition of the sugar acceptor and the regioselectivity of the products is poorly understood owing to low sequence identity around the acceptor‐binding region. PaGT3, a glycosyltransferase from the plant Phytolacca americana, can glycosylate a range of acceptors. To illustrate the structure–function relationship of PaGT3, its crystal structure was determined. The sugar‐donor and sugar‐acceptor binding pockets in PaGT3 were recognized by comparison of its structure with those of other UGTs. The key feature of PaGT3 was the presence of longer loop regions around the hydrophobic acceptor‐binding pocket, which resulted in a flexible and wider acceptor binding pocket. In this study, PaGT3 crystals were grown by co‐crystallization with 18‐crown‐6 ether or 15‐crown‐5 ether. The crown‐ether molecule in the asymmetric unit was observed to form a complex with a metal ion, which was coordinated on two sides by the main‐chain O atoms of Glu238 from two molecules of the protein. The crown ether–metal complex resembles a molecular glue that sticks two molecules of PaGT3 together to enhance crystal growth. Thus, this result provides an insight into the substrate‐recognition strategy in PaGT3 for the study of glycosyltransferases. Additionally, it is shown that crown ether–metal ion complexes can be used as a molecular glue for the crystallization of proteins.
中文翻译:

美洲植物糖基转移酶PaGT3的冠醚介导的晶体结构。
尿苷二磷酸糖基转移酶(UGTs)是普遍存在的酶,参与小分子的糖基化。随着糖基化改善疏水性化合物的水溶性和稳定性,人们对使用UGTs合成难溶性化合物的糖苷的兴趣正在增加。虽然存在植物次生产物糖基转移酶(PSPG)基序可以保护UGT中的糖供体识别,但由于受体周围的序列同一性较低,因此人们对糖受体的识别基础和产物的区域选择性知之甚少。结合区。Pa GT3是一种来自美洲植物疫霉的糖基转移酶,可以使一系列受体糖基化。为了说明结构与功能之间的关系Pa GT3,确定其晶体结构。通过将Pa GT3的糖供体和糖受体结合口袋与其他UGT的结构进行比较,可以识别出该口袋。Pa GT3的主要特征是在疏水性受体结合袋周围存在更长的环区,这导致了一个灵活且较宽的受体结合袋。在这项研究中,通过与18冠-6醚或15冠-5醚共结晶来生长Pa GT3晶体。观察到不对称单元中的冠醚分子与金属离子形成复合物,该复合物在两侧由来自两个蛋白质分子的Glu238的主链O原子配位。冠醚-金属配合物类似于分子胶,可黏附两个霸GT3一起增强晶体生长。因此,该结果为研究Pa GT3中糖基转移酶的底物识别策略提供了见识。此外,还表明冠醚-金属离子络合物可以用作蛋白质结晶的分子胶。
更新日期:2020-06-04
中文翻译:

美洲植物糖基转移酶PaGT3的冠醚介导的晶体结构。
尿苷二磷酸糖基转移酶(UGTs)是普遍存在的酶,参与小分子的糖基化。随着糖基化改善疏水性化合物的水溶性和稳定性,人们对使用UGTs合成难溶性化合物的糖苷的兴趣正在增加。虽然存在植物次生产物糖基转移酶(PSPG)基序可以保护UGT中的糖供体识别,但由于受体周围的序列同一性较低,因此人们对糖受体的识别基础和产物的区域选择性知之甚少。结合区。Pa GT3是一种来自美洲植物疫霉的糖基转移酶,可以使一系列受体糖基化。为了说明结构与功能之间的关系Pa GT3,确定其晶体结构。通过将Pa GT3的糖供体和糖受体结合口袋与其他UGT的结构进行比较,可以识别出该口袋。Pa GT3的主要特征是在疏水性受体结合袋周围存在更长的环区,这导致了一个灵活且较宽的受体结合袋。在这项研究中,通过与18冠-6醚或15冠-5醚共结晶来生长Pa GT3晶体。观察到不对称单元中的冠醚分子与金属离子形成复合物,该复合物在两侧由来自两个蛋白质分子的Glu238的主链O原子配位。冠醚-金属配合物类似于分子胶,可黏附两个霸GT3一起增强晶体生长。因此,该结果为研究Pa GT3中糖基转移酶的底物识别策略提供了见识。此外,还表明冠醚-金属离子络合物可以用作蛋白质结晶的分子胶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号