当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease.
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-06-04 , DOI: 10.1002/sctm.19-0327 Morris Losurdo 1 , Matteo Pedrazzoli 2 , Claudia D'Agostino 1 , Chiara A Elia 3, 4 , Francesca Massenzio 2 , Elena Lonati 1 , Mario Mauri 1 , Laura Rizzi 1 , Laura Molteni 1 , Elena Bresciani 1 , Erica Dander 5 , Giovanna D'Amico 5 , Alessandra Bulbarelli 1, 6 , Antonio Torsello 1 , Michela Matteoli 3, 7 , Mario Buffelli 2 , Silvia Coco 1, 6
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-06-04 , DOI: 10.1002/sctm.19-0327 Morris Losurdo 1 , Matteo Pedrazzoli 2 , Claudia D'Agostino 1 , Chiara A Elia 3, 4 , Francesca Massenzio 2 , Elena Lonati 1 , Mario Mauri 1 , Laura Rizzi 1 , Laura Molteni 1 , Elena Bresciani 1 , Erica Dander 5 , Giovanna D'Amico 5 , Alessandra Bulbarelli 1, 6 , Antonio Torsello 1 , Michela Matteoli 3, 7 , Mario Buffelli 2 , Silvia Coco 1, 6
Affiliation
|
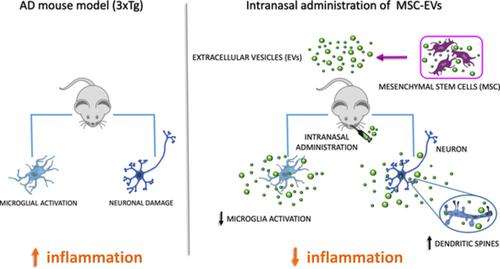
The critical role of neuroinflammation in favoring and accelerating the pathogenic process in Alzheimer's disease (AD) increased the need to target the cerebral innate immune cells as a potential therapeutic strategy to slow down the disease progression. In this scenario, mesenchymal stem cells (MSCs) have risen considerable interest thanks to their immunomodulatory properties, which have been largely ascribed to the release of extracellular vesicles (EVs), namely exosomes and microvesicles. Indeed, the beneficial effects of MSC‐EVs in regulating the inflammatory response have been reported in different AD mouse models, upon chronic intravenous or intracerebroventricular administration. In this study, we use the triple‐transgenic 3xTg mice showing for the first time that the intranasal route of administration of EVs, derived from cytokine‐preconditioned MSCs, was able to induce immunomodulatory and neuroprotective effects in AD. MSC‐EVs reached the brain, where they dampened the activation of microglia cells and increased dendritic spine density. MSC‐EVs polarized in vitro murine primary microglia toward an anti‐inflammatory phenotype suggesting that the neuroprotective effects observed in transgenic mice could result from a positive modulation of the inflammatory status. The possibility to administer MSC‐EVs through a noninvasive route and the demonstration of their anti‐inflammatory efficacy might accelerate the chance of a translational exploitation of MSC‐EVs in AD.
中文翻译:

鼻内递送间充质干细胞衍生的细胞外囊泡在阿尔茨海默病的 3xTg 模型中发挥免疫调节和神经保护作用。
神经炎症在促进和加速阿尔茨海默氏病(AD)的致病过程中发挥着关键作用,这增加了针对大脑先天免疫细胞作为减缓疾病进展的潜在治疗策略的需求。在这种情况下,间充质干细胞(MSC)由于其免疫调节特性而引起了相当大的兴趣,这在很大程度上归因于细胞外囊泡(EV)的释放,即外泌体和微泡。事实上,在不同的 AD 小鼠模型中,慢性静脉内或脑室内给药后,MSC-EV 在调节炎症反应方面的有益作用已被报道。在这项研究中,我们使用三重转基因 3xTg 小鼠,首次证明鼻内给药源自细胞因子预处理的 MSC 的 EV 能够在 AD 中诱导免疫调节和神经保护作用。 MSC-EV 到达大脑,抑制小胶质细胞的激活并增加树突棘密度。 MSC-EV 在体外使小鼠原代小胶质细胞极化为抗炎表型,这表明在转基因小鼠中观察到的神经保护作用可能是由于炎症状态的正向调节所致。通过非侵入性途径施用 MSC-EV 的可能性及其抗炎功效的证明可能会加速 MSC-EV 在 AD 中转化利用的机会。
更新日期:2020-06-04
中文翻译:

鼻内递送间充质干细胞衍生的细胞外囊泡在阿尔茨海默病的 3xTg 模型中发挥免疫调节和神经保护作用。
神经炎症在促进和加速阿尔茨海默氏病(AD)的致病过程中发挥着关键作用,这增加了针对大脑先天免疫细胞作为减缓疾病进展的潜在治疗策略的需求。在这种情况下,间充质干细胞(MSC)由于其免疫调节特性而引起了相当大的兴趣,这在很大程度上归因于细胞外囊泡(EV)的释放,即外泌体和微泡。事实上,在不同的 AD 小鼠模型中,慢性静脉内或脑室内给药后,MSC-EV 在调节炎症反应方面的有益作用已被报道。在这项研究中,我们使用三重转基因 3xTg 小鼠,首次证明鼻内给药源自细胞因子预处理的 MSC 的 EV 能够在 AD 中诱导免疫调节和神经保护作用。 MSC-EV 到达大脑,抑制小胶质细胞的激活并增加树突棘密度。 MSC-EV 在体外使小鼠原代小胶质细胞极化为抗炎表型,这表明在转基因小鼠中观察到的神经保护作用可能是由于炎症状态的正向调节所致。通过非侵入性途径施用 MSC-EV 的可能性及其抗炎功效的证明可能会加速 MSC-EV 在 AD 中转化利用的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号