当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unravelling the Mechanism of Rechargeable Aqueous Zn-MnO2 Batteries: Implementation of Charging Process by Electrodeposition of MnO2.
ChemSusChem ( IF 7.5 ) Pub Date : 2020-06-04 , DOI: 10.1002/cssc.202001216 Jie Yang 1, 2 , Jianyun Cao 3 , Yudong Peng 3 , Wenji Yang 3 , Suelen Barg 3 , Zhu Liu 3 , Ian A Kinloch 3 , Mark A Bissett 3 , Robert A W Dryfe 1, 2
ChemSusChem ( IF 7.5 ) Pub Date : 2020-06-04 , DOI: 10.1002/cssc.202001216 Jie Yang 1, 2 , Jianyun Cao 3 , Yudong Peng 3 , Wenji Yang 3 , Suelen Barg 3 , Zhu Liu 3 , Ian A Kinloch 3 , Mark A Bissett 3 , Robert A W Dryfe 1, 2
Affiliation
|
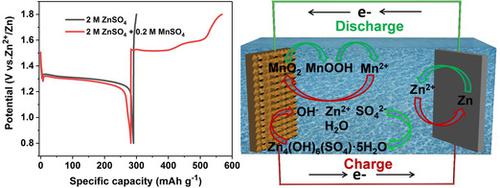
Poor cycling stability and mechanistic controversies have hindered the wider application of rechargeable aqueous Zn–MnO2 batteries. Herein, direct evidence was provided of the importance of Mn2+ in this type of battery by using a bespoke cell. Without pre‐addition of Mn2+, the cell exhibited an abnormal discharge–charge profile, meaning it functioned as a primary battery. By adjusting the Mn2+ content in the electrolyte, the cell recovered its charging ability through electrodeposition of MnO2. Additionally, a dynamic pH variation was observed during the discharge–charge process, with a precipitation of Zn4(OH)6(SO4)⋅5H2O buffering the pH of the electrolyte. Contrary to the conventional Zn2+ intercalation mechanism, MnO2 was first converted into MnOOH, which reverted to MnO2 through disproportionation, resulting in the dissolution of Mn2+. The charging process occurred by the electrodeposition of MnO2, thus improving the reversibility through the availability of Mn2+ ions in the solution.
中文翻译:

揭示可充电水系 Zn-MnO2 电池的机理:通过电解沉积 MnO2 实现充电过程。
较差的循环稳定性和机理争议阻碍了可充电水系Zn-MnO 2电池的更广泛应用。在此,通过使用定制电池提供了直接证据,证明了 Mn 2+在此类电池中的重要性。如果没有预先添加 Mn 2+,电池就会表现出异常的放电-充电曲线,这意味着它充当原电池。通过调节电解液中Mn 2+含量,电池通过电沉积MnO 2恢复充电能力。此外,在放电-充电过程中观察到动态pH值变化,Zn 4 (OH) 6 (SO 4 )⋅5H 2 O沉淀缓冲了电解质的pH值。与传统的Zn 2+嵌入机制相反,MnO 2首先转化为MnOOH,MnOOH通过歧化作用恢复为MnO 2 ,导致Mn 2+溶解。充电过程通过MnO 2的电沉积发生,从而通过溶液中Mn 2+离子的可用性来提高可逆性。
更新日期:2020-06-04
中文翻译:

揭示可充电水系 Zn-MnO2 电池的机理:通过电解沉积 MnO2 实现充电过程。
较差的循环稳定性和机理争议阻碍了可充电水系Zn-MnO 2电池的更广泛应用。在此,通过使用定制电池提供了直接证据,证明了 Mn 2+在此类电池中的重要性。如果没有预先添加 Mn 2+,电池就会表现出异常的放电-充电曲线,这意味着它充当原电池。通过调节电解液中Mn 2+含量,电池通过电沉积MnO 2恢复充电能力。此外,在放电-充电过程中观察到动态pH值变化,Zn 4 (OH) 6 (SO 4 )⋅5H 2 O沉淀缓冲了电解质的pH值。与传统的Zn 2+嵌入机制相反,MnO 2首先转化为MnOOH,MnOOH通过歧化作用恢复为MnO 2 ,导致Mn 2+溶解。充电过程通过MnO 2的电沉积发生,从而通过溶液中Mn 2+离子的可用性来提高可逆性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号