当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineered citrate synthase improves citramalic acid generation in Escherichia coli.
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-04 , DOI: 10.1002/bit.27450 Xianghao Wu 1 , D Brisbane Tovilla-Coutiño 1 , Mark A Eiteman 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-04 , DOI: 10.1002/bit.27450 Xianghao Wu 1 , D Brisbane Tovilla-Coutiño 1 , Mark A Eiteman 1
Affiliation
|
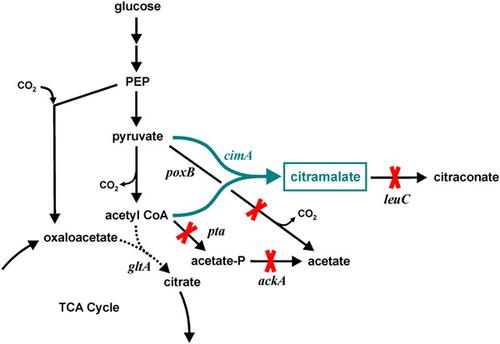
The microbial product citramalic acid (citramalate) serves as a five‐carbon precursor for the chemical synthesis of methacrylic acid. This biochemical is synthesized in Escherichia coli directly by the condensation of pyruvate and acetyl‐CoA via the enzyme citramalate synthase. The principal competing enzyme with citramalate synthase is citrate synthase, which mediates the condensation reaction of oxaloacetate and acetyl‐CoA to form citrate and begin the tricarboxylic acid cycle. A deletion in the gltA gene coding citrate synthase prevents acetyl‐CoA flux into the tricarboxylic acid cycle, and thus necessitates the addition of glutamate. In this study the E. coli citrate synthase was engineered to contain point mutations intended to reduce the enzyme's affinity for acetyl‐CoA, but not eliminate its activity. Cell growth, enzyme activity and citramalate production were compared in several variants in shake flasks and controlled fermenters. Citrate synthase GltA[F383M] not only facilitated cell growth without the presence of glutamate, but also improved the citramalate production by 125% compared with the control strain containing the native citrate synthase in batch fermentation. An exponential feeding strategy was employed in a fed‐batch process using MEC626/pZE12‐cimA harboring the GltA[F383M] variant, which generated over 60 g/L citramalate with a yield of 0.53 g citramalate/g glucose in 132 hr. These results demonstrate protein engineering can be used as an effective tool to redirect carbon flux by reducing enzyme activity and improve the microbial production of traditional commodity chemicals.
中文翻译:

工程柠檬酸合酶可改善大肠杆菌中柠檬酸的生成。
微生物产物柠檬酸(citramalate)是甲基丙烯酸化学合成的五碳前体。这种生化物质是通过丙酮酸和乙酰辅酶 A 经柠檬酸合酶缩合在大肠杆菌中直接合成的。柠檬酸合酶的主要竞争酶是柠檬酸合酶,它介导草酰乙酸和乙酰辅酶 A 的缩合反应形成柠檬酸并开始三羧酸循环。编码柠檬酸合酶的gltA基因缺失阻止乙酰辅酶 A 流入三羧酸循环,因此需要添加谷氨酸。在本研究中,大肠杆菌柠檬酸合酶被设计为包含点突变,旨在降低酶对乙酰辅酶 A 的亲和力,但不会消除其活性。在摇瓶和受控发酵罐中的几种变体中比较了细胞生长、酶活性和柠檬酸生产。柠檬酸合酶 GltA[F383M] 不仅在不存在谷氨酸的情况下促进细胞生长,而且在分批发酵中与含有天然柠檬酸合酶的对照菌株相比,柠檬酸产量提高了 125%。在使用 MEC626/pZE12-cimA 的分批补料过程中采用指数补料策略含有 GltA[F383M] 变体,其在 132 小时内产生超过 60 克/升的柠檬酸,产量为 0.53 克柠檬酸/克葡萄糖。这些结果表明,蛋白质工程可用作通过降低酶活性和改善传统商品化学品的微生物生产来重新定向碳通量的有效工具。
更新日期:2020-06-04
中文翻译:

工程柠檬酸合酶可改善大肠杆菌中柠檬酸的生成。
微生物产物柠檬酸(citramalate)是甲基丙烯酸化学合成的五碳前体。这种生化物质是通过丙酮酸和乙酰辅酶 A 经柠檬酸合酶缩合在大肠杆菌中直接合成的。柠檬酸合酶的主要竞争酶是柠檬酸合酶,它介导草酰乙酸和乙酰辅酶 A 的缩合反应形成柠檬酸并开始三羧酸循环。编码柠檬酸合酶的gltA基因缺失阻止乙酰辅酶 A 流入三羧酸循环,因此需要添加谷氨酸。在本研究中,大肠杆菌柠檬酸合酶被设计为包含点突变,旨在降低酶对乙酰辅酶 A 的亲和力,但不会消除其活性。在摇瓶和受控发酵罐中的几种变体中比较了细胞生长、酶活性和柠檬酸生产。柠檬酸合酶 GltA[F383M] 不仅在不存在谷氨酸的情况下促进细胞生长,而且在分批发酵中与含有天然柠檬酸合酶的对照菌株相比,柠檬酸产量提高了 125%。在使用 MEC626/pZE12-cimA 的分批补料过程中采用指数补料策略含有 GltA[F383M] 变体,其在 132 小时内产生超过 60 克/升的柠檬酸,产量为 0.53 克柠檬酸/克葡萄糖。这些结果表明,蛋白质工程可用作通过降低酶活性和改善传统商品化学品的微生物生产来重新定向碳通量的有效工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号