当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fabrication of magnetically recoverable and reusable MgFe2O4/Ag3PO4 composite for catalytic reduction of 4-Nitrophenol
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.solidstatesciences.2020.106302 P.N. Anantharamaiah , K.S. Manasa , Y.C. Sunil Kumar
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.solidstatesciences.2020.106302 P.N. Anantharamaiah , K.S. Manasa , Y.C. Sunil Kumar
|
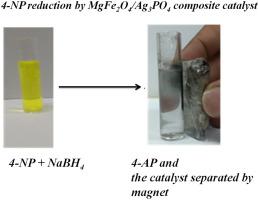
Abstract In recent years, an immense interest has been focused on to develop high-performance magnetic based nanocatalysts as they can be easily separated from the heterogeneous reaction mixture, without filtration/centrifugation process. Herein, a novel magnetically recoverable and reusable MgFe2O4/Ag3PO4 composite has been fabricated via a hydrothermal route to carry out 4-nitrophenol (4-NP) reduction reaction in aqueous medium. X-ray diffraction (XRD) patterns and Fourier transform infrared (FTIR) spectral analyses revealed the formation of a well-defined composite between magnetic (MgFe2O4) and non-magnetic (Ag3PO4) phases. The band gap of Ag3PO4 decreased from 2.31 eV to 1.71 eV after making composite with MgFe2O4. The fabricated composite is magnetic in nature with saturation magnetization (MS) of 15 emu/g. The prepared materials have been employed as catalysts to drive 4-NP reduction using the mild reducing agent sodium borohydride (NaBH4) and the progress of the catalytic reaction was ascertained using UV–visible spectrophotometry. Results show that the fabricated MgFe2O4/Ag3PO4 composite demonstrated superior catalytic performance over the individual components (MgFe2O4 and Ag3PO4) of the composite. Moreover, the composite completed the 4-nitrophenol reduction reaction in 1 min whereas the Ag3PO4 achieved the same reduction reaction in 15 min, despite all the catalytic reduction reactions were performed under the identical conditions. Enhanced catalytic performance of the MgFe2O4/Ag3PO4 magnetic composite can be attributed to strong electronic coupling between MgFe2O4 and Ag3PO4 that facilitates the electrons transfer from borohydride to nitrophenol and thereby catalytic reduction reaction occurs. Furthermore, the composite catalyst was found to be phase stable even after 5 consecutive recycles.
中文翻译:

用于催化还原 4-硝基苯酚的磁性可回收和可重复使用的 MgFe2O4/Ag3PO4 复合材料的制备
摘要 近年来,人们对开发高性能磁性纳米催化剂产生了极大的兴趣,因为它们可以很容易地从多相反应混合物中分离,无需过滤/离心过程。在此,通过水热途径制备了一种新型可磁性回收和可重复使用的 MgFe2O4/Ag3PO4 复合材料,以在水性介质中进行 4-硝基苯酚 (4-NP) 还原反应。X 射线衍射 (XRD) 图案和傅里叶变换红外 (FTIR) 光谱分析表明,磁性 (MgFe2O4) 和非磁性 (Ag3PO4) 相之间形成了明确的复合材料。与MgFe2O4复合后,Ag3PO4的带隙从2.31eV降低到1.71eV。制造的复合材料具有磁性,饱和磁化强度 (MS) 为 15 emu/g。制备的材料已被用作催化剂,使用温和的还原剂硼氢化钠 (NaBH4) 驱动 4-NP 还原,并使用紫外-可见分光光度法确定催化反应的进程。结果表明,制备的 MgFe2O4/Ag3PO4 复合材料表现出优于复合材料的单个组分(MgFe2O4 和 Ag3PO4)的催化性能。此外,复合材料在 1 分钟内完成了 4-硝基苯酚的还原反应,而 Ag3PO4 在 15 分钟内完成了相同的还原反应,尽管所有催化还原反应都是在相同的条件下进行的。MgFe2O4/Ag3PO4 磁性复合材料的催化性能增强可归因于 MgFe2O4 和 Ag3PO4 之间的强电子耦合,促进电子从硼氢化物转移到硝基苯酚,从而发生催化还原反应。此外,发现复合催化剂即使在连续 5 次循环后仍是相稳定的。
更新日期:2020-08-01
中文翻译:

用于催化还原 4-硝基苯酚的磁性可回收和可重复使用的 MgFe2O4/Ag3PO4 复合材料的制备
摘要 近年来,人们对开发高性能磁性纳米催化剂产生了极大的兴趣,因为它们可以很容易地从多相反应混合物中分离,无需过滤/离心过程。在此,通过水热途径制备了一种新型可磁性回收和可重复使用的 MgFe2O4/Ag3PO4 复合材料,以在水性介质中进行 4-硝基苯酚 (4-NP) 还原反应。X 射线衍射 (XRD) 图案和傅里叶变换红外 (FTIR) 光谱分析表明,磁性 (MgFe2O4) 和非磁性 (Ag3PO4) 相之间形成了明确的复合材料。与MgFe2O4复合后,Ag3PO4的带隙从2.31eV降低到1.71eV。制造的复合材料具有磁性,饱和磁化强度 (MS) 为 15 emu/g。制备的材料已被用作催化剂,使用温和的还原剂硼氢化钠 (NaBH4) 驱动 4-NP 还原,并使用紫外-可见分光光度法确定催化反应的进程。结果表明,制备的 MgFe2O4/Ag3PO4 复合材料表现出优于复合材料的单个组分(MgFe2O4 和 Ag3PO4)的催化性能。此外,复合材料在 1 分钟内完成了 4-硝基苯酚的还原反应,而 Ag3PO4 在 15 分钟内完成了相同的还原反应,尽管所有催化还原反应都是在相同的条件下进行的。MgFe2O4/Ag3PO4 磁性复合材料的催化性能增强可归因于 MgFe2O4 和 Ag3PO4 之间的强电子耦合,促进电子从硼氢化物转移到硝基苯酚,从而发生催化还原反应。此外,发现复合催化剂即使在连续 5 次循环后仍是相稳定的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号