Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-06-03 , DOI: 10.1016/j.omtm.2020.06.002 Sem J Aronson 1 , Robert S Bakker 1 , Sascha Moenis 1 , Remco van Dijk 1 , Giulia Bortolussi 2 , Fanny Collaud 3 , Xiaoxia Shi 1 , Suzanne Duijst 1 , Lysbeth Ten Bloemendaal 1 , Giuseppe Ronzitti 3 , Andrés F Muro 2 , Federico Mingozzi 3 , Ulrich Beuers 1 , Piter J Bosma 1
|
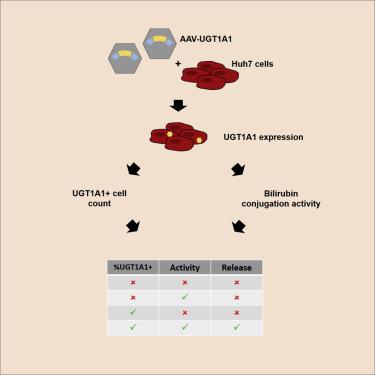
Potency assessment of clinical-grade vector lots is crucial to support adeno-associated virus (AAV) vector release and is required for future marketing authorization. We have developed and validated a cell-based, quantitative potency assay that detects both transgenic expression and activity of an AAV8-hUGT1A1 vector, which is currently under clinical evaluation for the treatment of Crigler-Najjar syndrome. Potency of AAV8-hUGT1A1 was evaluated in vitro. After transduction of human hepatoma 7 (Huh7) cells, transgene-positive cells were quantified using flow cytometry and transgenic activity by a bilirubin conjugation assay. The in vitro potency of various AAV8-hUGT1A1 batches was compared with their potency in vivo. After AAV8-hUGT1A1 transduction, quantification of UGT1A1-expressing cells shows a linear dose-response relation (R2 = 0.98) with adequate intra-assay and inter-day reproducibility (coefficient of variation [CV] = 11.0% and 22.6%, respectively). In accordance, bilirubin conjugation shows a linear dose-response relation (R2 = 0.99) with adequate intra- and inter-day reproducibility in the low dose range (CV = 15.7% and 19.7%, respectively). Both in vitro potency assays reliably translate to in vivo efficacy of AAV8-hUGT1A1 vector lots. The described cell-based potency assay for AAV8-hUGT1A1 adequately determines transgenic UGT1A1 expression and activity, which is consistent with in vivo efficacy. This novel approach is suited for the determination of vector lot potency to support clinical-grade vector release.
中文翻译:

编码UGT1A1转基因的腺相关病毒载体的定量体外效能测定。
临床级载体批次的效能评估对于支持腺相关病毒(AAV)载体的释放至关重要,并且是未来上市授权所必需的。我们已经开发并验证了一种基于细胞的定量效能测定方法,该方法可检测AAV8-h UGT1A1载体的转基因表达和活性,目前正在临床评估中用于治疗Crigler-Najjar综合征。在体外评估了AAV8-h UGT1A1的效力。转导人肝癌7(Huh7)细胞后,使用流式细胞仪对转基因阳性细胞进行定量,并通过胆红素结合测定法对转基因活性进行定量。比较了各种AAV8-hUGT1A1批次的体外效能与它们在体内的效能。在AAV8-h UGT1A1转导后,定量表达UGT1A1的细胞显示出线性的剂量反应关系(R 2 = 0.98),具有足够的测定内和日间重现性(变异系数[CV] = 11.0%和22.6%,分别)。因此,胆红素结合表现出线性的剂量-反应关系(R 2 = 0.99),在低剂量范围内(CV = 15.7%和19.7%),具有足够的日内和日间重现性。两种体外效价测定均可靠地转化为AAV8-h UGT1A1载体批次的体内功效。所述的AAV8-h UGT1A1的基于细胞的效能测定足够确定转基因UGT1A1的表达和活性,这与体内功效一致。这种新颖的方法适用于确定载体批次效价以支持临床级载体的释放。










































 京公网安备 11010802027423号
京公网安备 11010802027423号