Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-06-04 , DOI: 10.1016/j.bmcl.2020.127305 Rajan Giri 1 , Hari K Namballa 2 , Ananta Sarker 2 , Ian Alberts 3 , Wayne W Harding 4
|
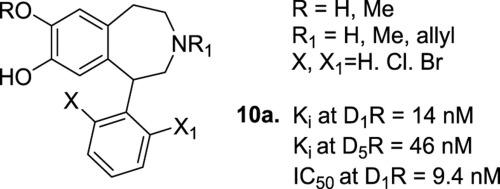
A series of 1-phenylbenzazepines containing bromine or chlorine substituents at the ortho position of the appended phenyl ring (2′-monosubstituted or 2′,6′- disubstituted patterns) were synthesized and evaluated for affinity towards dopamine D1R, D2R and D5R. As is typical of the 1-phenylbenzazepine scaffold, the compounds displayed selectivity towards D1R and D5R; analogs generally lacked affinity for D2R. Interestingly, 2′,6′-dichloro substituted analogs showed modest D5R versus D1R selectivity whereas this selectivity was reversed in compounds with a 2′-halo substitution pattern. Compound 10a was identified as a D1R antagonist (Ki = 14 nM; IC50 = 9.4 nM).
中文翻译:

环 C 邻卤化 1-苯基苯并氮杂的合成和多巴胺受体药理学评价。
合成了一系列在附加苯环的邻位(2'-单取代或 2',6'-双取代模式)含有溴或氯取代基的 1-苯基苯并氮杂并评估了对多巴胺 D 1 R、D 2 R 的亲和力和 D 5 R。作为典型的 1-苯基苯并氮杂支架,这些化合物对 D 1 R 和 D 5 R表现出选择性;类似物通常对 D 2 R缺乏亲和力。有趣的是,2',6'-二氯取代的类似物显示出适度的 D 5 R 与 D 1 R 选择性,而这种选择性在具有 2'-卤素取代模式的化合物中相反。化合物10a被鉴定为D 1 R拮抗剂(K i = 14 nM;IC 50 = 9.4 nM)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号