当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Disulfides as mercapto-precursors in nucleophilic ring opening reaction of polymeric epoxides: establishing equimolar stoichiometric conditions in a thiol-epoxy 'click' reaction.
Chemical Communications ( IF 4.9 ) Pub Date : 2020-06-03 , DOI: 10.1039/d0cc02601h Taejun Eom 1 , Anzar Khan
Chemical Communications ( IF 4.9 ) Pub Date : 2020-06-03 , DOI: 10.1039/d0cc02601h Taejun Eom 1 , Anzar Khan
Affiliation
|
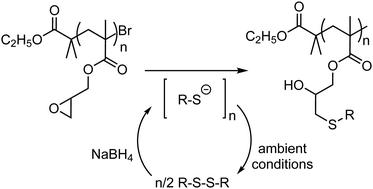
The base-catalyzed oxirane ring opening reaction with thiol nucleophiles is frequently employed for post-polymerization modification of polymeric glycidyl scaffolds. Due to various beneficial attributes, it is often referred to as a ‘click’ reaction. However, the tendency of the free thiol molecules to undergo oxidative dimerization through the formation of a disulfide bond under ambient conditions results in partial consumption of the sulfhydryl precursors. Therefore, an excess of the thiol precursors is typically used to counterbalance the side-reaction. This violates the equimolar stoichiometry conditions required for ‘click’ reactions in the context of polymer synthesis. Here, we show that commercially available disulfides can be used to generate the necessary thiolate nucleophiles in situ through the reduction of the SS-bond with sodium borohydride. Such activation strategy eliminates the sulfhydryl oxidation mechanism to disulfides and ensures that the post-synthesis functionalization of epoxy polymers can be performed under equimolar ‘click’ conditions.
中文翻译:

二硫化物在聚合物环氧化物的亲核开环反应中作为巯基前体:在硫醇-环氧“点击”反应中建立等摩尔化学计量条件。
与硫醇亲核试剂的碱催化的环氧乙烷开环反应经常用于聚合缩水甘油基支架的后聚合修饰。由于各种有益的属性,它通常被称为“点击”反应。但是,游离硫醇分子在环境条件下通过形成二硫键而经历氧化二聚的趋势导致巯基前体的部分消耗。因此,通常使用过量的硫醇前体来平衡副反应。这违反了在聚合物合成的情况下“点击”反应所需的等摩尔化学计量条件。在这里,我们表明可商购的二硫化物可用于原位生成必要的硫醇盐亲核体通过用硼氢化钠还原SS键来实现。这种活化策略消除了巯基氧化成二硫化物的机理,并确保了环氧聚合物的合成后官能化可以在等摩尔“点击”条件下进行。
更新日期:2020-07-07
中文翻译:

二硫化物在聚合物环氧化物的亲核开环反应中作为巯基前体:在硫醇-环氧“点击”反应中建立等摩尔化学计量条件。
与硫醇亲核试剂的碱催化的环氧乙烷开环反应经常用于聚合缩水甘油基支架的后聚合修饰。由于各种有益的属性,它通常被称为“点击”反应。但是,游离硫醇分子在环境条件下通过形成二硫键而经历氧化二聚的趋势导致巯基前体的部分消耗。因此,通常使用过量的硫醇前体来平衡副反应。这违反了在聚合物合成的情况下“点击”反应所需的等摩尔化学计量条件。在这里,我们表明可商购的二硫化物可用于原位生成必要的硫醇盐亲核体通过用硼氢化钠还原SS键来实现。这种活化策略消除了巯基氧化成二硫化物的机理,并确保了环氧聚合物的合成后官能化可以在等摩尔“点击”条件下进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号