当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Retrosynthetic Analysis of α-Alkenyl-β-Diketones: Regio- and Stereoselective Two-Step Synthesis of Highly Arylated Representatives from Acetylenes, Ketones, and Acyl Chlorides.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.joc.0c00768 Dmitrii A Shabalin 1 , Elena V Ivanova 1 , Igor' A Ushakov 1 , Elena Yu Schmidt 1 , Boris A Trofimov 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.joc.0c00768 Dmitrii A Shabalin 1 , Elena V Ivanova 1 , Igor' A Ushakov 1 , Elena Yu Schmidt 1 , Boris A Trofimov 1
Affiliation
|
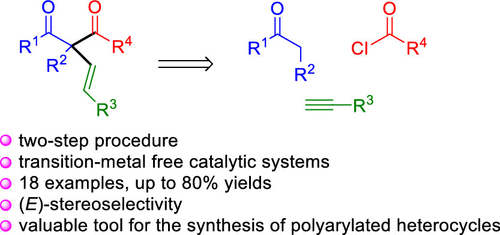
Highly arylated α-alkenyl-β-diketones are synthesized via a two-step sequence consisting of (i) potassium tert-butoxide/DMSO-catalyzed (E)-stereoselective C–H functionalization of ketones with acetylenes followed by (ii) magnesium bromide etherate/DIPEA-soft enolization of the formed β,γ-unsaturated ketones and regioselective acylation with acyl chlorides. The method is compatible with a broad range of substrates and shown to be applicable as an intermediate stage in the construction of polyarylated heterocycles.
中文翻译:

α-链烯基-β-二酮的逆合成分析:乙炔基,酮和酰氯的高度丙烯酸化的区域和立体选择性两步合成法。
高度芳基化的α-烯基-β-二酮是通过两步序列合成的:(i)叔丁醇钾/ DMSO催化的乙炔经酮的(E)立体选择性CH官能化,然后是(ii)溴化镁生成的β,γ-不饱和酮的醚化/ DIPEA软化烯化反应,并用酰氯进行区域选择性酰化。该方法与广泛的底物相容,并且显示出可作为构建聚芳基杂环的中间阶段。
更新日期:2020-07-02
中文翻译:

α-链烯基-β-二酮的逆合成分析:乙炔基,酮和酰氯的高度丙烯酸化的区域和立体选择性两步合成法。
高度芳基化的α-烯基-β-二酮是通过两步序列合成的:(i)叔丁醇钾/ DMSO催化的乙炔经酮的(E)立体选择性CH官能化,然后是(ii)溴化镁生成的β,γ-不饱和酮的醚化/ DIPEA软化烯化反应,并用酰氯进行区域选择性酰化。该方法与广泛的底物相容,并且显示出可作为构建聚芳基杂环的中间阶段。











































 京公网安备 11010802027423号
京公网安备 11010802027423号