当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Emissive ortho-Donor–Acceptor Triarylboranes: Impact of Boryl Acceptors on Luminescence Properties
Organometallics ( IF 2.5 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.organomet.0c00185 Juhee Kim 1 , Taehwan Lee 1 , Ji Yeon Ryu 2 , Young Hoon Lee 1 , Junseong Lee 2 , Jaehoon Jung 1 , Min Hyung Lee 1
Organometallics ( IF 2.5 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.organomet.0c00185 Juhee Kim 1 , Taehwan Lee 1 , Ji Yeon Ryu 2 , Young Hoon Lee 1 , Junseong Lee 2 , Jaehoon Jung 1 , Min Hyung Lee 1
Affiliation
|
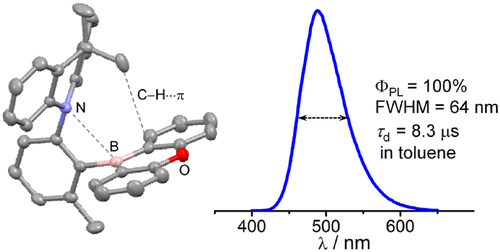
Highly emissive ortho-donor (D)–acceptor (A) triarylboranes with different boryl acceptors are proposed. 9-Boraanthryl (1), 10H-phenoxaboryl (2), and dimesitylboryl (BMes2, 3) acceptor moieties were combined with a fixed 9,9-dimethylacridine (DMAC) donor to produce ortho-D–A compounds. X-ray diffraction and solution nuclear magnetic resonance spectroscopy analyses reveal the sterically congested and highly twisted D–A structure. Weak N···B and C–H···π nonbonding interactions between DMAC and boryl moieties are persistently present in the solid state of cyclic boryl-containing 1 and 2, as judged from short contacts of approximately 2.80–2.84 Å between the N and B atoms and of approximately 2.86–3.00 Å between the CH3 group and the boryl plane. This feature is further supported by computational studies for intramolecular nonbonding interactions in 1 and 2. All compounds are highly emissive with photoluminescence quantum yields (PLQYs) approaching 100% and exhibit strong thermally activated delayed fluorescence in solution and in the rigid state. Notably, a decrease in PLQY with an increase in the polarity of the solvent is less sensitive for the cyclic boryl-containing 1 and 2. In particular, 1 and 2 exhibit blue-shifted fluorescence with narrow bandwidths in both toluene and a PMMA film. Theoretical studies further support the twisted structure, effective HOMO–LUMO separation, and the small singlet–triplet energy splitting, with all corroborating the observed photophysical properties.
中文翻译:

高发射邻-供体-受体三芳基硼烷:硼基受体对发光性能的影响
提出了具有不同硼基受体的高发射邻位给体(D)-受体(A)三芳基硼烷。9- Boraanthryl(1),10 ħ -phenoxaboryl(2),和dimesitylboryl(BMES 2,3)受体部分用固定的9,9- dimethylacridine(DMAC)供体以产生组合的邻-D-A的化合物。X射线衍射和溶液核磁共振波谱分析揭示了空间拥挤和高度扭曲的D–A结构。DMAC和硼烷基部分之间的弱N··B和C–H···π非键相互作用始终存在于含环硼基1和2的固态中根据N和B原子之间约2.80–2.84Å的短接触以及CH 3基团与硼基平面之间约2.86–3.00Å的短接触来判断。对1和2中分子内非键相互作用的计算研究进一步支持了此功能。所有化合物均具有高发射率,光致发光量子产率(PLQYs)接近100%,并在溶液和刚性状态下均显示出强烈的热活化延迟荧光。值得注意的是,随着溶剂极性的增加,PLQY的降低对含环状硼基的1和2的敏感性较低。特别是1和2在甲苯和PMMA膜中均显示出窄带宽的蓝移荧光。理论研究进一步支持扭曲的结构,有效的HOMO-LUMO分离以及小的单重态-三重态能量分裂,所有这些都证实了所观察到的光物理性质。
更新日期:2020-06-23
中文翻译:

高发射邻-供体-受体三芳基硼烷:硼基受体对发光性能的影响
提出了具有不同硼基受体的高发射邻位给体(D)-受体(A)三芳基硼烷。9- Boraanthryl(1),10 ħ -phenoxaboryl(2),和dimesitylboryl(BMES 2,3)受体部分用固定的9,9- dimethylacridine(DMAC)供体以产生组合的邻-D-A的化合物。X射线衍射和溶液核磁共振波谱分析揭示了空间拥挤和高度扭曲的D–A结构。DMAC和硼烷基部分之间的弱N··B和C–H···π非键相互作用始终存在于含环硼基1和2的固态中根据N和B原子之间约2.80–2.84Å的短接触以及CH 3基团与硼基平面之间约2.86–3.00Å的短接触来判断。对1和2中分子内非键相互作用的计算研究进一步支持了此功能。所有化合物均具有高发射率,光致发光量子产率(PLQYs)接近100%,并在溶液和刚性状态下均显示出强烈的热活化延迟荧光。值得注意的是,随着溶剂极性的增加,PLQY的降低对含环状硼基的1和2的敏感性较低。特别是1和2在甲苯和PMMA膜中均显示出窄带宽的蓝移荧光。理论研究进一步支持扭曲的结构,有效的HOMO-LUMO分离以及小的单重态-三重态能量分裂,所有这些都证实了所观察到的光物理性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号