当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ketone Reductase Biocatalysis in the Synthesis of Chiral Intermediates Toward Generic Active Pharmaceutical Ingredients
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.oprd.0c00120 Marina Y. Raynbird 1 , Joanne B. Sampson 1 , Dan A. Smith 1 , Siân M. Forsyth 1 , Jonathan D. Moseley 1 , Andrew S. Wells 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.oprd.0c00120 Marina Y. Raynbird 1 , Joanne B. Sampson 1 , Dan A. Smith 1 , Siân M. Forsyth 1 , Jonathan D. Moseley 1 , Andrew S. Wells 2
Affiliation
|
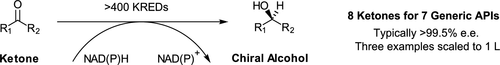
A range of generic active pharmaceutical ingredients were examined for potential chiral alcohol motifs and derivatives within their structures that could be employed as key synthetic intermediates. For seven generic active pharmaceutical ingredients (APIs), eight precursor ketones were acquired and then subjected to reduction by >400 commercially available ketone reductases from different suppliers. Positive screening results were achieved for five ketones screened, with multiple ketone reductases available for each successful ketone. Selectivity was typically >99.5% ee in most cases, including for the opposite enantiomer. The three best examples were then optimized and quickly scaled up to 1 L scale in high conversion and isolated yield while retaining selectivity of >99.5% ee for the desired chiral alcohol enantiomer. This work illustrates that where a wide range of enzymes are available, productive enzymes to give either alcohol enantiomer can be readily identified for many ketones and rapidly scaled up to produce chiral alcohols. This approach is particularly applicable to generating chiral API intermediates.
中文翻译:

酮还原酶对手性中间体合成通用活性药物成分的生物催化
检查了一系列通用活性药物成分,以了解其结构内可能用作关键合成中间体的手性醇基序和衍生物。对于七个通用活性药物成分(API),获取了八个前体酮,然后用来自不同供应商的> 400多种市售酮还原酶进行还原。筛选出的五个酮获得了积极的筛选结果,每个成功的酮都有多种酮还原酶可用。在大多数情况下,包括相对的对映异构体,选择性通常> 99.5%ee。然后对三个最佳实例进行优化,并以高转化率和分离的收率快速放大至1 L规模,同时保持对所需手性醇对映体的选择性> 99.5%ee。这项工作说明,在可以使用多种酶的地方,可以很容易地从许多酮中识别出产生任何一种醇对映体的生产酶,并迅速扩大规模以生产手性醇。此方法特别适用于生成手性API中间体。
更新日期:2020-06-19
中文翻译:

酮还原酶对手性中间体合成通用活性药物成分的生物催化
检查了一系列通用活性药物成分,以了解其结构内可能用作关键合成中间体的手性醇基序和衍生物。对于七个通用活性药物成分(API),获取了八个前体酮,然后用来自不同供应商的> 400多种市售酮还原酶进行还原。筛选出的五个酮获得了积极的筛选结果,每个成功的酮都有多种酮还原酶可用。在大多数情况下,包括相对的对映异构体,选择性通常> 99.5%ee。然后对三个最佳实例进行优化,并以高转化率和分离的收率快速放大至1 L规模,同时保持对所需手性醇对映体的选择性> 99.5%ee。这项工作说明,在可以使用多种酶的地方,可以很容易地从许多酮中识别出产生任何一种醇对映体的生产酶,并迅速扩大规模以生产手性醇。此方法特别适用于生成手性API中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号