当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective and Stereospecific Rhodium-Catalyzed Allylic Cyanomethylation with an Acetonitrile Equivalent: Construction of Acyclic β-Quaternary Stereogenic Nitriles
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-02 , DOI: 10.1021/jacs.0c02316 Mai-Jan Tom 1 , P Andrew Evans 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-02 , DOI: 10.1021/jacs.0c02316 Mai-Jan Tom 1 , P Andrew Evans 1, 2
Affiliation
|
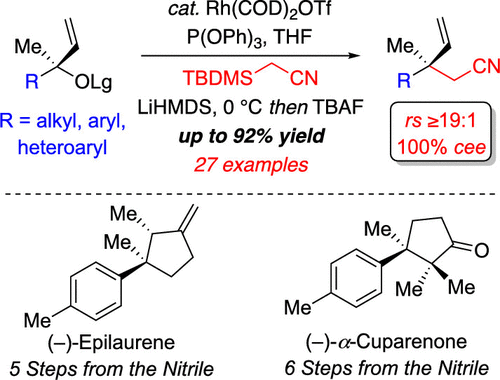
A highly regioselective and stereospecific rhodium-catalyzed cyanomethylation of tertiary allylic carbonates for the construction of acyclic β-quaternary stereogenic nitriles is described. This protocol represents the first example of a metal-catalyzed allylic substitution reaction using a triorganosilyl-stabilized acetonitrile anion, which permits access to several carbonyl derivatives that are challenging to prepare using conventional pronucleophiles. The synthetic utility of the stereospecific cyanomethylation is further exemplified through the construction of the intermediate utilized in the total synthesis of both (+)-epilaurene and (+)-α-cuparenone.
中文翻译:

区域选择性和立体特异性铑催化烯丙基氰甲基化与乙腈等价物:构建无环 β-四元立体腈
描述了一种高度区域选择性和立体有择的铑催化的叔烯丙基碳酸酯氰甲基化,用于构建无环 β-四元立体腈。该协议代表了使用三有机甲硅烷基稳定的乙腈阴离子进行金属催化烯丙基取代反应的第一个例子,它允许访问使用传统亲核试剂制备具有挑战性的几种羰基衍生物。立体特异性氰甲基化的合成效用进一步通过构建用于 (+)-表烯丙烯和 (+)-α-cuparenone 全合成的中间体来举例说明。
更新日期:2020-06-02
中文翻译:

区域选择性和立体特异性铑催化烯丙基氰甲基化与乙腈等价物:构建无环 β-四元立体腈
描述了一种高度区域选择性和立体有择的铑催化的叔烯丙基碳酸酯氰甲基化,用于构建无环 β-四元立体腈。该协议代表了使用三有机甲硅烷基稳定的乙腈阴离子进行金属催化烯丙基取代反应的第一个例子,它允许访问使用传统亲核试剂制备具有挑战性的几种羰基衍生物。立体特异性氰甲基化的合成效用进一步通过构建用于 (+)-表烯丙烯和 (+)-α-cuparenone 全合成的中间体来举例说明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号