当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis of Inhibition of Insulin-Regulated Aminopeptidase by a Macrocyclic Peptidic Inhibitor.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-06-02 , DOI: 10.1021/acsmedchemlett.0c00172 Anastasia Mpakali 1 , Emmanuel Saridakis 1 , Petros Giastas 1 , Zachary Maben 2 , Lawrence J Stern 2 , Mats Larhed 3 , Mathias Hallberg 4 , Efstratios Stratikos 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-06-02 , DOI: 10.1021/acsmedchemlett.0c00172 Anastasia Mpakali 1 , Emmanuel Saridakis 1 , Petros Giastas 1 , Zachary Maben 2 , Lawrence J Stern 2 , Mats Larhed 3 , Mathias Hallberg 4 , Efstratios Stratikos 1
Affiliation
|
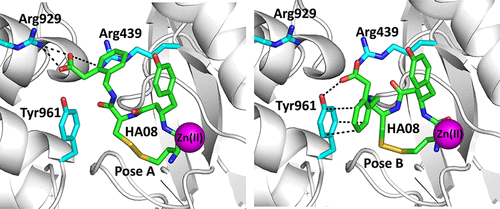
Insulin-regulated aminopeptidase (IRAP) is a transmembrane zinc metallopeptidase with many important biological functions and an emerging pharmacological target. Although previous structural studies have given insight on how IRAP recognizes linear peptides, how it recognizes its physiological cyclic ligands remains elusive. Here, we report the first crystal structure of IRAP with the macrocyclic peptide inhibitor HA08 that combines structural elements from angiotensin IV and the physiological substrates oxytocin and vasopressin. The compound is found in the catalytic site in a near canonical substrate-like configuration and inhibits by a competitive mechanism. Comparison with previously solved structures of IRAP along with small-angle X-ray scattering experiments suggests that IRAP is in an open conformation in solution but undergoes a closing conformational change upon inhibitor binding. Stabilization of the closed conformation in combination with catalytic water exclusion by the tightly juxtaposed GAMEN loop is proposed as a mechanism of inhibition.
中文翻译:

大环肽抑制剂抑制胰岛素调节的氨基肽酶的结构基础。
胰岛素调节氨肽酶(IRAP)是一种跨膜锌金属肽酶,具有许多重要的生物学功能和新兴的药理学靶点。尽管之前的结构研究已经深入了解 IRAP 如何识别线性肽,但它如何识别其生理环状配体仍然难以捉摸。在这里,我们报道了 IRAP 与大环肽抑制剂 HA08 的第一个晶体结构,该抑制剂结合了血管紧张素 IV 的结构元件以及生理底物催产素和加压素。该化合物在催化位点中以接近经典底物的结构存在,并通过竞争机制进行抑制。与先前解析的 IRAP 结构以及小角 X 射线散射实验的比较表明,IRAP 在溶液中处于开放构象,但在抑制剂结合后经历闭合构象变化。封闭构象的稳定与紧密并置的 GAMEN 环催化水排除相结合被认为是一种抑制机制。
更新日期:2020-07-09
中文翻译:

大环肽抑制剂抑制胰岛素调节的氨基肽酶的结构基础。
胰岛素调节氨肽酶(IRAP)是一种跨膜锌金属肽酶,具有许多重要的生物学功能和新兴的药理学靶点。尽管之前的结构研究已经深入了解 IRAP 如何识别线性肽,但它如何识别其生理环状配体仍然难以捉摸。在这里,我们报道了 IRAP 与大环肽抑制剂 HA08 的第一个晶体结构,该抑制剂结合了血管紧张素 IV 的结构元件以及生理底物催产素和加压素。该化合物在催化位点中以接近经典底物的结构存在,并通过竞争机制进行抑制。与先前解析的 IRAP 结构以及小角 X 射线散射实验的比较表明,IRAP 在溶液中处于开放构象,但在抑制剂结合后经历闭合构象变化。封闭构象的稳定与紧密并置的 GAMEN 环催化水排除相结合被认为是一种抑制机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号