当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst‐Free Preparation of Perfluoroalkyl‐Phosphoryl Substituted Furans from 1‐Perfluoroalkyl 1,3‐Diketones in Two Steps
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1002/adsc.202000534 Stanislav V. Lozovskiy 1 , Aleksander V. Vasilyev 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1002/adsc.202000534 Stanislav V. Lozovskiy 1 , Aleksander V. Vasilyev 1, 2
Affiliation
|
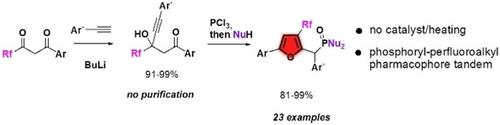
A two‐step catalyst‐free procedure for the construction of 3‐perfluoroalkyl‐2‐(phosphoryl)methyl furans from commercially available 1‐perfluoroalkyl 1,3‐diketones is reported. Propargylic alcohols, prepared by direct alkynylation of the diketones, react with PCl3 via [2,3]‐sigmatropic rearrangement – enolate cyclization sequence, leading to multifunctional furans. The reaction mechanism is supported by DFT study. In addition, the bioactivity modifying effect of phosphoryl group on 3‐perfluoroalkyl furans is depicted.
中文翻译:

分两步从1-全氟烷基1,3-二酮无催化剂制备全氟烷基-磷酰基取代的呋喃
据报道,从市售的1-全氟烷基1,3-二酮类化合物制备三-全氟烷基-2-(磷酰基)甲基呋喃需要两步操作,无需催化剂。通过二酮的直接烷基化制备的炔丙醇通过[2,3] -sigmatropic重排(烯醇化环化序列)与PCl 3反应,生成多功能呋喃。反应机理得到DFT研究的支持。此外,还描述了磷酸基对3-全氟烷基呋喃的生物活性改性作用。
更新日期:2020-08-04
中文翻译:

分两步从1-全氟烷基1,3-二酮无催化剂制备全氟烷基-磷酰基取代的呋喃
据报道,从市售的1-全氟烷基1,3-二酮类化合物制备三-全氟烷基-2-(磷酰基)甲基呋喃需要两步操作,无需催化剂。通过二酮的直接烷基化制备的炔丙醇通过[2,3] -sigmatropic重排(烯醇化环化序列)与PCl 3反应,生成多功能呋喃。反应机理得到DFT研究的支持。此外,还描述了磷酸基对3-全氟烷基呋喃的生物活性改性作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号