当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zirconium‐Salan Catalyzed Enantioselective α‐Hydroxylation of β‐Keto Esters
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1002/adsc.202000290 Jie Chen 1 , Haiyang Gu 1 , Xueying Zhu 1 , Wonwoo Nam 2 , Bin Wang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1002/adsc.202000290 Jie Chen 1 , Haiyang Gu 1 , Xueying Zhu 1 , Wonwoo Nam 2 , Bin Wang 1
Affiliation
|
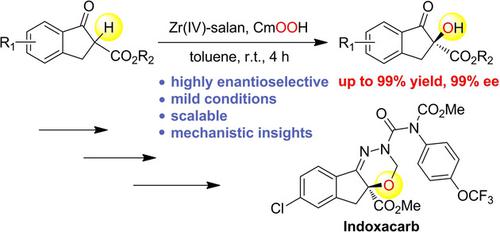
We report herein the development of enantioselective and scalable α‐hydroxylation of 1‐indanone‐derived β‐keto esters by Zr(IV) complexes bearing readily available C2‐symmetric salan ligands and cumene hydroperoxide as an oxidant, affording synthetically valuable hydroxylation products in high yields (up to 99%) with excellent enantioselectivities (up to 99% ee) under mild reaction conditions. In mechanistic studies, we have shown that (1) a Zr(IV)‐salan complex was generated in situ as the active catalyst responsible for the chiral induction, (2) the transfer of the electrophilic fragment from cumene hydroperoxide to the Zr(IV)‐bound enolate was accompanied by a heterolytic O−O bond cleavage, and (3) the formation of hydrogen bond between the amine hydrogen atom(s) of the salan ligand and the hydroxy group of cumene hydroperoxide was significant for stabilizing the stereocontrolled transition state and improving the enantioselectivity.
中文翻译:

锆-Salan催化β-酮基酯的对映选择性α-羟基化
我们在这里报告通过带有容易获得的C 2的Zr(IV)配合物对1-茚满酮衍生的β-酮酯的对映选择性和可扩展性α-羟基化的发展。对称的Salan配体和枯烯氢过氧化物作为氧化剂,在温和的反应条件下,可提供高收率(高达99%)和优异的对映选择性(高达99%ee)的合成有价值的羟基化产物。在机理研究中,我们表明(1)原位生成Zr(IV)-salan络合物作为负责手性诱导的活性催化剂,(2)亲电片段从氢过氧化枯烯向Zr(IV)的转移)结合的烯醇化物伴随有杂化的O-O键断裂,(3)salan配体的胺氢原子与氢过氧化枯烯的羟基之间形成氢键对于稳定立体控制的跃迁非常重要并提高对映选择性。
更新日期:2020-07-29
中文翻译:

锆-Salan催化β-酮基酯的对映选择性α-羟基化
我们在这里报告通过带有容易获得的C 2的Zr(IV)配合物对1-茚满酮衍生的β-酮酯的对映选择性和可扩展性α-羟基化的发展。对称的Salan配体和枯烯氢过氧化物作为氧化剂,在温和的反应条件下,可提供高收率(高达99%)和优异的对映选择性(高达99%ee)的合成有价值的羟基化产物。在机理研究中,我们表明(1)原位生成Zr(IV)-salan络合物作为负责手性诱导的活性催化剂,(2)亲电片段从氢过氧化枯烯向Zr(IV)的转移)结合的烯醇化物伴随有杂化的O-O键断裂,(3)salan配体的胺氢原子与氢过氧化枯烯的羟基之间形成氢键对于稳定立体控制的跃迁非常重要并提高对映选择性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号