当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stem cell mobilization with plerixafor and healing of diabetic ischemic wounds: A phase IIa, randomized, double-blind, placebo-controlled trial.
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-06-02 , DOI: 10.1002/sctm.20-0020 Benedetta Maria Bonora 1, 2 , Roberta Cappellari 1, 2 , Marta Mazzucato 1 , Mauro Rigato 1, 3 , Marco Grasso 1 , Mirko Menegolo 4 , Andrea Bruttocao 1 , Angelo Avogaro 1 , Gian Paolo Fadini 1, 2, 3
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-06-02 , DOI: 10.1002/sctm.20-0020 Benedetta Maria Bonora 1, 2 , Roberta Cappellari 1, 2 , Marta Mazzucato 1 , Mauro Rigato 1, 3 , Marco Grasso 1 , Mirko Menegolo 4 , Andrea Bruttocao 1 , Angelo Avogaro 1 , Gian Paolo Fadini 1, 2, 3
Affiliation
|
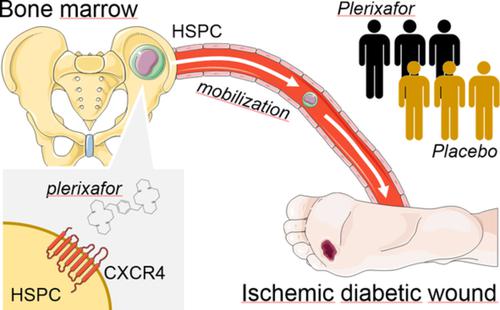
Bone marrow‐derived cells contribute to tissue repair, but traffic of hematopoietic stem/progenitor cells (HSPCs) is impaired in diabetes. We therefore tested whether HSPC mobilization with the CXCR4 antagonist plerixafor improved healing of ischemic diabetic wounds. This was a pilot, phase IIa, double‐blind, randomized, placebo‐controlled trial (NCT02790957). Patients with diabetes with ischemic wounds were randomized to receive a single subcutaneous injection of plerixafor or saline on top of standard medical and surgical therapy. The primary endpoint was complete healing at 6 months. Secondary endpoints were wound size, transcutaneous oxygen tension (TcO2), ankle‐brachial index (ABI), amputations, and HSPC mobilization. Twenty‐six patients were enrolled: 13 received plerixafor and 13 received placebo. Patients were 84.6% males, with a mean age of 69 years. HSPC mobilization was successful in all patients who received plerixafor. The trial was terminated after a preplanned interim analysis of 50% of the target population showed a significantly lower healing rate in the plerixafor vs the placebo group. In the final analysis data set, the rate of complete healing was 38.5% in the plerixafor group vs 69.2% in the placebo group (chi‐square P = .115). Wound size tended to be larger in the plerixafor group for the entire duration of observation. No significant difference was noted for the change in TcO2 and ABI or in amputation rates. No other safety concern emerged. In conclusion, successful HSPC mobilization with plerixafor did not improve healing of ischemic diabetic wounds. Contrary to what was expected, outside the context of hematological disorders, mobilization of diabetic HSPCs might exert adverse effects on wound healing.
中文翻译:

使用plerixafor进行干细胞动员和糖尿病缺血性伤口的愈合:IIa期随机,双盲,安慰剂对照试验。
骨髓来源的细胞有助于组织修复,但造血干/祖细胞(HSPC)的运输在糖尿病中受损。因此,我们测试了使用CXCR4拮抗剂plerixa进行HSPC动员是否可改善缺血性糖尿病伤口的愈合。这是一项IIa期,双盲,随机,安慰剂对照试验(NCT02790957)。患有缺血性伤口的糖尿病患者被随机分配在标准的医学和外科治疗基础上接受皮下注射普乐沙福或生理盐水。主要终点为6个月时完全愈合。次要终点是伤口大小,经皮氧气张力(TcO 2),踝臂指数(ABI),截肢术和HSPC动员。纳入26位患者:13例接受普立卡福,13例接受安慰剂。患者为84.6%的男性,平均年龄为69岁。HSPC动员在所有接受plerixafor的患者中均获得成功。在对50%的目标人群进行预先计划的中期分析后,该试验被终止,该试验显示plerixafor与安慰剂组的治愈率显着降低。在最终分析数据集中,普乐力福组的完全治愈率为38.5%,而安慰剂组为69.2%(卡方P = .115)。在整个观察期间,plerixafor组的伤口大小倾向于更大。TcO 2的变化无明显差异和ABI或截肢率。没有其他安全隐患出现。综上所述,使用plerixafor成功进行HSPC动员并不能改善缺血性糖尿病伤口的愈合。与预期相反,在血液学疾病之外,糖尿病性HSPC的动员可能会对伤口愈合产生不利影响。
更新日期:2020-06-02
中文翻译:

使用plerixafor进行干细胞动员和糖尿病缺血性伤口的愈合:IIa期随机,双盲,安慰剂对照试验。
骨髓来源的细胞有助于组织修复,但造血干/祖细胞(HSPC)的运输在糖尿病中受损。因此,我们测试了使用CXCR4拮抗剂plerixa进行HSPC动员是否可改善缺血性糖尿病伤口的愈合。这是一项IIa期,双盲,随机,安慰剂对照试验(NCT02790957)。患有缺血性伤口的糖尿病患者被随机分配在标准的医学和外科治疗基础上接受皮下注射普乐沙福或生理盐水。主要终点为6个月时完全愈合。次要终点是伤口大小,经皮氧气张力(TcO 2),踝臂指数(ABI),截肢术和HSPC动员。纳入26位患者:13例接受普立卡福,13例接受安慰剂。患者为84.6%的男性,平均年龄为69岁。HSPC动员在所有接受plerixafor的患者中均获得成功。在对50%的目标人群进行预先计划的中期分析后,该试验被终止,该试验显示plerixafor与安慰剂组的治愈率显着降低。在最终分析数据集中,普乐力福组的完全治愈率为38.5%,而安慰剂组为69.2%(卡方P = .115)。在整个观察期间,plerixafor组的伤口大小倾向于更大。TcO 2的变化无明显差异和ABI或截肢率。没有其他安全隐患出现。综上所述,使用plerixafor成功进行HSPC动员并不能改善缺血性糖尿病伤口的愈合。与预期相反,在血液学疾病之外,糖尿病性HSPC的动员可能会对伤口愈合产生不利影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号