当前位置:
X-MOL 学术
›
J. Biomed. Mater. Res. Part A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
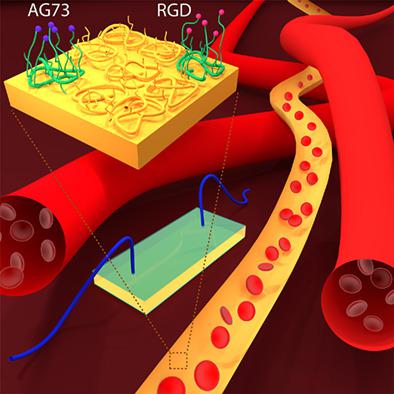
Biomaterials functionalized with nanoclusters of integrin- and syndecan-binding ligands improve cell adhesion and mechanosensing under shear flow conditions.
Journal of Biomedical Materials Research Part A ( IF 4.9 ) Pub Date : 2020-06-03 , DOI: 10.1002/jbm.a.37024 Fatemeh Karimi 1, 2, 3 , Varsha Jagannath Thombare 4 , Craig A Hutton 4 , Andrea J O'Connor 1 , Greg G Qiao 2 , Daniel E Heath 1
Journal of Biomedical Materials Research Part A ( IF 4.9 ) Pub Date : 2020-06-03 , DOI: 10.1002/jbm.a.37024 Fatemeh Karimi 1, 2, 3 , Varsha Jagannath Thombare 4 , Craig A Hutton 4 , Andrea J O'Connor 1 , Greg G Qiao 2 , Daniel E Heath 1
Affiliation
|
We have engineered biomaterials that display nanoclusters of ligands that bind both integrin and syndecan‐4 cell receptors. These surfaces regulate cell behaviors under static conditions including adhesion, spreading, actin stress fiber formation, and migration. The syndecan‐4 receptors are also critical mediators of cellular mechanotransduction. In this contribution we assess whether this novel class of materials can regulate the response of cells to applied mechanical stimulation, using the shear stress imparted by laminar fluid flow as a model stimulus. Specifically, we assess endothelial cell detachment due to flow, cell alignment due to flow, and cell adhesion from the flowing fluid. A high degree of cell retention was observed on surfaces containing integrin‐binding ligands or a mixed population of integrin‐ and syndecan‐binding ligands. However, the presence of both ligand types was necessary for the cells to align in the direction of flow. These results imply that integrin engagement is necessary for adhesion strength, but engagement of both receptor types aids in appropriate mechanotransduction. Additionally, it was found that surfaces functionalized with both ligand types were able to scavenge a larger number of cells from flow, and to do so at a faster rate, compared to surfaces functionalized with only integrin‐ or syndecan‐binding ligands. These results show that interfaces functionalized with both integrin‐ and syndecan‐binding ligands regulate a significant range of biophysical cell behaviors in response to shear stress.
中文翻译:

用整合素和多糖结合配体的纳米团簇功能化的生物材料在剪切流条件下改善细胞粘附和机械感应。
我们设计了生物材料,可以展示结合整联蛋白和 Syndecan-4 细胞受体的纳米配体团簇。这些表面在静态条件下调节细胞行为,包括粘附、扩散、肌动蛋白应力纤维形成和迁移。syndecan-4 受体也是细胞机械转导的关键介质。在这项贡献中,我们使用层流流动赋予的剪切应力作为模型刺激来评估这种新型材料是否可以调节细胞对施加的机械刺激的反应。具体来说,我们评估了由于流动引起的内皮细胞脱离、由于流动引起的细胞排列以及流动流体中的细胞粘附。在含有整合素结合配体或整合素和合成聚糖结合配体的混合群的表面上观察到高度的细胞滞留。然而,两种配体类型的存在对于细胞在流动方向上对齐是必要的。这些结果意味着整联蛋白的参与对于粘附强度是必要的,但两种受体类型的参与有助于适当的机械转导。此外,发现与仅使用整合素或多聚体结合配体功能化的表面相比,使用两种配体类型功能化的表面能够从流动中清除更多的细胞,并且以更快的速度清除。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。这些结果意味着整联蛋白的参与对于粘附强度是必要的,但两种受体类型的参与有助于适当的机械转导。此外,发现与仅使用整合素或多聚体结合配体功能化的表面相比,使用两种配体类型功能化的表面能够从流动中清除更多的细胞,并且以更快的速度清除。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。这些结果意味着整联蛋白的参与对于粘附强度是必要的,但两种受体类型的参与有助于适当的机械转导。此外,发现与仅使用整合素或多聚体结合配体功能化的表面相比,使用两种配体类型功能化的表面能够从流动中清除更多的细胞,并且以更快的速度清除。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。与仅使用整合素或多聚体结合配体功能化的表面相比,这样做的速度更快。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。与仅使用整合素或多聚体结合配体功能化的表面相比,这样做的速度更快。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。
更新日期:2020-06-03
中文翻译:

用整合素和多糖结合配体的纳米团簇功能化的生物材料在剪切流条件下改善细胞粘附和机械感应。
我们设计了生物材料,可以展示结合整联蛋白和 Syndecan-4 细胞受体的纳米配体团簇。这些表面在静态条件下调节细胞行为,包括粘附、扩散、肌动蛋白应力纤维形成和迁移。syndecan-4 受体也是细胞机械转导的关键介质。在这项贡献中,我们使用层流流动赋予的剪切应力作为模型刺激来评估这种新型材料是否可以调节细胞对施加的机械刺激的反应。具体来说,我们评估了由于流动引起的内皮细胞脱离、由于流动引起的细胞排列以及流动流体中的细胞粘附。在含有整合素结合配体或整合素和合成聚糖结合配体的混合群的表面上观察到高度的细胞滞留。然而,两种配体类型的存在对于细胞在流动方向上对齐是必要的。这些结果意味着整联蛋白的参与对于粘附强度是必要的,但两种受体类型的参与有助于适当的机械转导。此外,发现与仅使用整合素或多聚体结合配体功能化的表面相比,使用两种配体类型功能化的表面能够从流动中清除更多的细胞,并且以更快的速度清除。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。这些结果意味着整联蛋白的参与对于粘附强度是必要的,但两种受体类型的参与有助于适当的机械转导。此外,发现与仅使用整合素或多聚体结合配体功能化的表面相比,使用两种配体类型功能化的表面能够从流动中清除更多的细胞,并且以更快的速度清除。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。这些结果意味着整联蛋白的参与对于粘附强度是必要的,但两种受体类型的参与有助于适当的机械转导。此外,发现与仅使用整合素或多聚体结合配体功能化的表面相比,使用两种配体类型功能化的表面能够从流动中清除更多的细胞,并且以更快的速度清除。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。与仅使用整合素或多聚体结合配体功能化的表面相比,这样做的速度更快。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。与仅使用整合素或多聚体结合配体功能化的表面相比,这样做的速度更快。这些结果表明,用整合素和多聚体结合配体功能化的界面可调节响应剪切应力的大量生物物理细胞行为。



























 京公网安备 11010802027423号
京公网安备 11010802027423号