当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiomerically Pure Quinoline-Based κ-Opioid Receptor Agonists: Chemoenzymatic Synthesis and Pharmacological Evaluation.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-06-03 , DOI: 10.1002/cmdc.202000300 Benedikt Martin 1 , Dirk Schepmann 1 , Freddy A Bernal 2 , Thomas J Schmidt 2 , Tao Che 3 , Karin Loser 4, 5 , Bernhard Wünsch 1, 5
ChemMedChem ( IF 3.6 ) Pub Date : 2020-06-03 , DOI: 10.1002/cmdc.202000300 Benedikt Martin 1 , Dirk Schepmann 1 , Freddy A Bernal 2 , Thomas J Schmidt 2 , Tao Che 3 , Karin Loser 4, 5 , Bernhard Wünsch 1, 5
Affiliation
|
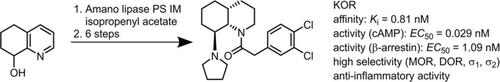
Racemic K‐opioid receptor (KOR) agonist 2‐(3,4‐dichlorophenyl)‐1‐[(4aRS ,8SR ,8aSR )‐8‐(pyrrolidin‐1‐yl)‐3,4,4a,5,6,7,8,8a‐octahydroquinolin‐1(2H )‐yl]ethan‐1‐one ((±)‐4 ) was prepared in a diastereoselective synthesis. The first key step of the synthesis was the diastereoselective hydrogenation of the silyl ether of 1,2,3,4‐tetrahydroquinoin‐8‐ol ((±)‐9 ) to afford cis ,cis‐configured perhydroquinoline derivative (±)‐10 . Removal of the TBDMS protecting group led to a β‐aminoalcohol that reacted with SO2Cl2 to form an oxathiazolidine. Nucleophilic substitution with pyrrolidine resulted in the desired cis ,trans‐configured perhydroquinoline upon inversion of the configuration. In order to obtain enantiomerically pure KOR agonists 4 (99.8 % ee ) and ent‐4 (99.0 % ee ), 1,2,3,4‐tetrahydroquinolin‐8‐ols (R )‐8 (99.1 % ee ) and (S )‐8 (98.4 % ee ) were resolved by an enantioselective acetylation catalyzed by Amano lipase PS‐IM. The absolute configuration was determined by CD spectroscopy. The 4aR ,8S ,8aS‐configured enantiomer 4 showed sub‐nanomolar KOR affinity (K i=0.81 nM), which is more than 200 times higher than the KOR affinity of its enantiomer ent‐4 . In the cAMP assay and the Tango β‐arrestin‐2 recruitment assay, 4 behaved as a KOR agonist. Upon incubation of human macrophages, human dendritic cells, and mouse myeloid immune cells with 4 , the number of cells expressing co‐stimulatory receptor CD86 and proinflammatory cytokines interleukin 6 and tumor necrosis factor α was significantly reduced; this indicates the strong anti‐inflammatory activity of 4 . The anti‐inflammatory effects correlated well with the KOR affinity: (4aR ,8S ,8aS )‐4 was slightly more potent than the racemic mixture (±)‐4 , and the distomer ent‐4 was almost inactive.
中文翻译:

对映体纯喹啉基 κ-阿片受体激动剂:化学酶合成和药理学评价。
外消旋K-阿片受体(KOR)激动剂2-(3,4-二氯苯基)-1-[(4a RS ,8 SR ,8a SR )-8-(吡咯烷-1-基)-3,4,4a,5 ,6,7,8,8a-八氢喹啉-1(2 H )-基]乙烷-1-酮((±) -4 )是通过非对映选择性合成制备的。合成的第一个关键步骤是1,2,3,4-四氢醌-8-醇((±)-9 )的甲硅烷基醚的非对映选择性氢化,得到顺式、顺式构型的全氢喹啉衍生物(±) -10。去除 TBDMS 保护基团会产生 β-氨基醇,该醇与 SO 2 Cl 2反应形成恶噻唑烷。吡咯烷的亲核取代在构型反转后产生了所需的顺式、反式构型的全氢喹啉。为了获得对映体纯的 KOR 激动剂4 (99.8 % ee ) 和ent ‐ 4 (99.0 % ee ),1,2,3,4-四氢喹啉-8-醇 ( R )‐ 8 (99.1 % ee ) 和 ( S )‐ 8 (98.4 % ee ) 通过天野脂肪酶 PS-IM 催化的对映选择性乙酰化进行解析。通过CD光谱测定绝对构型。4a R , 8 S ,8a S构型的对映体4显示出亚纳摩尔 KOR 亲和力(K i =0.81 nM),比其对映体4的 KOR 亲和力高 200 倍以上。在 cAMP 测定和 Tango β-arrestin-2 招募测定中,4表现为 KOR 激动剂。将人巨噬细胞、人树突状细胞和小鼠骨髓免疫细胞与4一起孵育后,表达共刺激受体 CD86 和促炎细胞因子白细胞介素 6 和肿瘤坏死因子 α 的细胞数量显着减少;这表明4具有很强的抗炎活性。抗炎作用与 KOR 亲和力密切相关:(4a R ,8 S ,8a S )- 4比外消旋混合物 (±)- 4稍强,而反异构体- 4几乎没有活性。
更新日期:2020-08-05
中文翻译:

对映体纯喹啉基 κ-阿片受体激动剂:化学酶合成和药理学评价。
外消旋K-阿片受体(KOR)激动剂2-(3,4-二氯苯基)-1-[(4a RS ,8 SR ,8a SR )-8-(吡咯烷-1-基)-3,4,4a,5 ,6,7,8,8a-八氢喹啉-1(2 H )-基]乙烷-1-酮((±) -4 )是通过非对映选择性合成制备的。合成的第一个关键步骤是1,2,3,4-四氢醌-8-醇((±)-9 )的甲硅烷基醚的非对映选择性氢化,得到顺式、顺式构型的全氢喹啉衍生物(±) -10。去除 TBDMS 保护基团会产生 β-氨基醇,该醇与 SO 2 Cl 2反应形成恶噻唑烷。吡咯烷的亲核取代在构型反转后产生了所需的顺式、反式构型的全氢喹啉。为了获得对映体纯的 KOR 激动剂4 (99.8 % ee ) 和ent ‐ 4 (99.0 % ee ),1,2,3,4-四氢喹啉-8-醇 ( R )‐ 8 (99.1 % ee ) 和 ( S )‐ 8 (98.4 % ee ) 通过天野脂肪酶 PS-IM 催化的对映选择性乙酰化进行解析。通过CD光谱测定绝对构型。4a R , 8 S ,8a S构型的对映体4显示出亚纳摩尔 KOR 亲和力(K i =0.81 nM),比其对映体4的 KOR 亲和力高 200 倍以上。在 cAMP 测定和 Tango β-arrestin-2 招募测定中,4表现为 KOR 激动剂。将人巨噬细胞、人树突状细胞和小鼠骨髓免疫细胞与4一起孵育后,表达共刺激受体 CD86 和促炎细胞因子白细胞介素 6 和肿瘤坏死因子 α 的细胞数量显着减少;这表明4具有很强的抗炎活性。抗炎作用与 KOR 亲和力密切相关:(4a R ,8 S ,8a S )- 4比外消旋混合物 (±)- 4稍强,而反异构体- 4几乎没有活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号