当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scalable Photoelectrochemical Dehydrogenative Cross-Coupling of Heteroarenes with Aliphatic C-H Bonds.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-06-02 , DOI: 10.1002/anie.202005724 Pin Xu 1 , Peng-Yu Chen 1 , Hai-Chao Xu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-06-02 , DOI: 10.1002/anie.202005724 Pin Xu 1 , Peng-Yu Chen 1 , Hai-Chao Xu 1
Affiliation
|
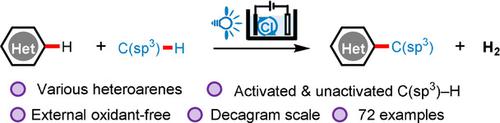
Heteroarenes are structural motifs found in many bioactive compounds and functional materials. Dehydrogenative cross‐coupling of heteroarenes with aliphatic C−H bonds provides straightforward access to functionalized heteroarenes from readily available materials. Established methods employ stoichiometric chemical oxidants under conditions of heating or light irradiation. By merging electrochemistry and photochemistry, we have achieved efficient photoelectrochemical dehydrogenative cross‐coupling of heteroarenes and C(sp3)−H donors through H2 evolution, without the addition of metal catalysts or chemical oxidants. Mechanistically, the C(sp3)−H donor is converted to a nucleophilic carbon radical through H‐atom transfer with chlorine atom, which is produced by light irradiation of anodically generated Cl2 from Cl−. The carbon radical then undergoes radical substitution to the heteroarene to afford alkylated heteroarene products.
中文翻译:

杂芳烃与脂肪族CH键的可扩展光电化学脱氢交叉偶联。
杂芳烃是在许多生物活性化合物和功能材料中发现的结构基序。杂芳烃与脂肪族CH键的脱氢交叉偶联可直接从容易获得的材料中获得官能化的杂芳烃。既定的方法在加热或光照条件下采用化学计量的化学氧化剂。通过合并电化学和光化学,我们已经实现了杂芳烃和C(sp 3)-H供体通过H 2释放的有效光电化学脱氢交叉偶联,而无需添加金属催化剂或化学氧化剂。从机械上讲,C(sp 3)-H供体被转化为亲核碳通过与氯原子,这是由阳极产生的Cl光照射产生的H-原子转移自由基2选自Cl - 。然后碳自由基被杂芳基自由基取代以提供烷基化的杂芳烃产物。
更新日期:2020-08-10
中文翻译:

杂芳烃与脂肪族CH键的可扩展光电化学脱氢交叉偶联。
杂芳烃是在许多生物活性化合物和功能材料中发现的结构基序。杂芳烃与脂肪族CH键的脱氢交叉偶联可直接从容易获得的材料中获得官能化的杂芳烃。既定的方法在加热或光照条件下采用化学计量的化学氧化剂。通过合并电化学和光化学,我们已经实现了杂芳烃和C(sp 3)-H供体通过H 2释放的有效光电化学脱氢交叉偶联,而无需添加金属催化剂或化学氧化剂。从机械上讲,C(sp 3)-H供体被转化为亲核碳通过与氯原子,这是由阳极产生的Cl光照射产生的H-原子转移自由基2选自Cl - 。然后碳自由基被杂芳基自由基取代以提供烷基化的杂芳烃产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号