Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.omtm.2020.05.034 Jeong-A Lim 1 , Su Jin Choi 1 , Fengqin Gao 1 , Priya S Kishnani 1 , Baodong Sun 1
|
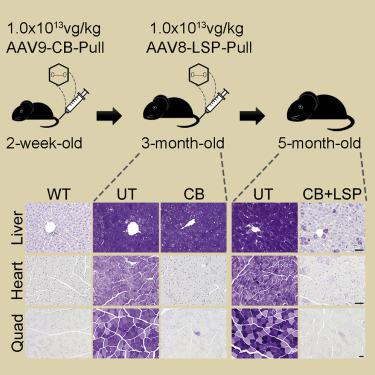
Glycogen storage disease type III (GSD III) is an inherited disorder caused by a deficiency of glycogen debranching enzyme (GDE), which results in the accumulation of abnormal glycogen (limit dextrin) in the cytoplasm of liver, heart, and skeletal muscle cells. Currently, there is no curative treatment for this disease. Gene therapy with adeno-associated virus (AAV) provides an optimal treatment approach for monogenic diseases like GSD III. However, the 4.6 kb human GDE cDNA is too large to be packaged into a single AAV vector due to its small carrying capacity. To overcome this limitation, we tested a new gene therapy approach in GSD IIIa mice using an AAV vector ubiquitously expressing a smaller bacterial GDE, Pullulanase, whose cDNA is 2.2 kb. Intravenous injection of the AAV vector (AAV9-CB-Pull) into 2-week-old GSD IIIa mice blocked glycogen accumulation in both cardiac and skeletal muscles, but not in the liver, accompanied by the improvement of muscle functions. Subsequent treatment with a liver-restricted AAV vector (AAV8-LSP-Pull) reduced liver glycogen content by 75% and reversed hepatic fibrosis while maintaining the effect of AAV9-CB-Pull treatment on heart and skeletal muscle. Our results suggest that AAV-mediated gene therapy with Pullulanase is a possible treatment for GSD III.
中文翻译:

GSD III的一种新型基因治疗方法,使用的AAV载体编码细菌糖原解支酶。
糖原贮积病III型(GSD III)是由糖原解支酶(GDE)缺乏引起的遗传性疾病,导致肝,心脏和骨骼肌细胞质中异常糖原(极限糊精)的积累。目前,尚无该病的治疗方法。腺相关病毒(AAV)的基因疗法为GSD III等单基因疾病提供了最佳治疗方法。然而,由于其小的携带能力,4.6kb的人GDE cDNA太大而不能包装到单个AAV载体中。为了克服这一局限性,我们使用了无处不在的AAV载体在GSD IIIa小鼠中测试了一种新的基因治疗方法,该载体普遍表达较小的细菌GDE支链淀粉酶,其cDNA为2.2 kb。将AAV载体(AAV9-CB-Pull)静脉内注射到2周大的GSD IIIa小鼠中,阻断了糖原在心脏和骨骼肌中的蓄积,但在肝脏中没有,从而伴随着肌肉功能的改善。随后用限制肝的AAV载体(AAV8-LSP-Pull)治疗可将肝糖原含量降低75%,并逆转肝纤维化,同时保持AAV9-CB-Pull治疗对心脏和骨骼肌的作用。我们的结果表明,用支链淀粉酶进行AAV介导的基因治疗可能是GSD III的一种治疗方法。随后用限制肝的AAV载体(AAV8-LSP-Pull)治疗可将肝糖原含量降低75%,并逆转肝纤维化,同时保持AAV9-CB-Pull治疗对心脏和骨骼肌的作用。我们的结果表明,用支链淀粉酶进行AAV介导的基因治疗可能是GSD III的一种治疗方法。随后用限制肝的AAV载体(AAV8-LSP-Pull)治疗可将肝糖原含量降低75%,并逆转肝纤维化,同时保持AAV9-CB-Pull治疗对心脏和骨骼肌的作用。我们的结果表明,用支链淀粉酶进行AAV介导的基因治疗可能是GSD III的一种治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号