International Immunopharmacology ( IF 4.8 ) Pub Date : 2020-06-03 , DOI: 10.1016/j.intimp.2020.106647 Jing Huang 1 , Lan Wang 2 , Chuanfei Yu 2 , Zhihao Fu 2 , Chunyu Liu 2 , Hongmei Zhang 3 , Kaiqin Wang 2 , Xiao Guo 2 , Junzhi Wang 1
|
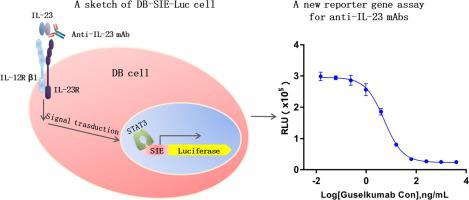
Environmental disturbances may result in dysregulation of interleukin-23 (IL-23), which is a crucial modulator of immunity. Several therapeutic monoclonal antibodies (mAbs) have been developed for treating IL-23-related autoimmune inflammation, such as ustekinumab, guselkumab, tildrakizumab, and risankizumab. Accurate bioactivity determination of therapeutic mAbs is essential for their quality control and clinical application. However, the current methods are tedious and complicated. In the present study, we employed low-background lentivirus carrying sis-inducible element (SIE)-driven firefly luciferase to generate a stable DB-SIE-Luc cell line that expresses endogenous IL-23 receptors and developed a sensitive and straightforward reporter gene assay (RGA) based on DB-SIE-Luc cells. After the optimization of various assay parameters, we set up a bioassay with the best fit of a four-parameter model and an appropriate signal-to-noise ratio (SNR) for bioactivity determination of guselkumab. We further verified the excellent assay performance characteristics of our RGA, including specificity, linearity, accuracy, precision, and stability, according to ICH-Q2. Taken together, we established a reliable and robust cell-based RGA, which potentially serves as a valubale alternative bioactivity determination assay for the release control and stability study of anti-IL-23 mAbs.
中文翻译:

用于测量治疗性抗白介素23单克隆抗体生物活性的可靠的基于细胞的报告基因测定的表征。
环境干扰可能会导致白细胞介素23(IL-23)失调,白细胞介素23是免疫力的关键调节因子。已经开发出几种用于治疗IL-23相关自身免疫炎症的治疗性单克隆抗体(mAb),例如ustekinumab,guselkumab,tildrakizumab和risankizumab。准确测定治疗性单克隆抗体的生物活性对于其质量控制和临床应用至关重要。但是,当前的方法乏味且复杂。在本研究中,我们采用携带sis诱导元件(SIE)驱动的萤火虫荧光素酶的低背景慢病毒来生成表达内源性IL-23受体的稳定DB-SIE-Luc细胞系,并开发了敏感而直接的报告基因检测(RGA)基于DB-SIE-Luc单元。优化各种测定参数后,我们建立了一种最适合四参数模型并具有合适的信噪比(SNR)的生物测定法,用于测定古斯库单抗的生物活性。根据ICH-Q2,我们进一步验证了我们RGA出色的测定性能,包括特异性,线性,准确性,精密度和稳定性。综上所述,我们建立了可靠且强大的基于细胞的RGA,它可作为抗IL-23 mAb的释放控制和稳定性研究的重要替代生物活性测定方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号