Biomaterials ( IF 12.8 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.biomaterials.2020.120166 Jing Yan 1 , Qinghua Wu 1 , Ziyin Zhao 1 , Jianhua Wu 2 , Huan Ye 1 , Qiujun Liang 1 , Zhuchao Zhou 2 , Mengying Hou 1 , Xudong Li 1 , Yong Liu 1 , Lichen Yin 1
|
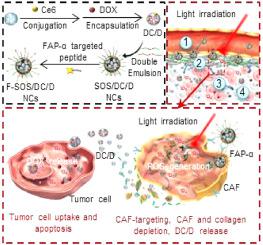
The anticancer performance of nanomedicine is largely impeded by insufficient intratumoral penetration. Herein, tumor microenvironment (TME)-amendatory and self-adaptive nanoclusters (NCs) capable of cancer-associated fibroblasts (CAFs) depletion and size/charge conversion were engineered to mediate light-assisted, hierarchical intratumoral penetration. Particularly, large-sized NCs (~50 nm) were prepared via self-assembly of FAP-α-targeting peptide-modified, 1O2-sensitive polymers, which were further used to envelope small-sized dendrimer (~5 nm) conjugated with Ce6 and loaded with DOX (DC/D). After systemic administration, the NCs efficiently targeted CAFs and generated lethal levels of 1O2 upon light irradiation, which depleted CAFs and concomitantly dissociated the NCs to liberate small-sized, positively charged DC/D. Such stroma attenuation and NCs transformation collectively facilitated the delivery of DC/D into deeper regions of CAF-rich tumors, where DOX and 1O2 provoked synergistic anti-cancer efficacies. This study provides an effective approach to facilitate the tumor penetration of nanomedicine by concurrently and spatiotemporally reconfiguring the nano-properties and remodeling the TME.
中文翻译:

基于肿瘤微环境(TME)修正和自适应聚合物纳米团簇的光辅助分级肿瘤内穿透和程序化抗肿瘤治疗。
纳米药物的抗癌性能在很大程度上受到肿瘤内穿透不足的阻碍。本文中,设计了能够与癌症相关的成纤维细胞(CAF)耗竭和大小/电荷转换的肿瘤微环境(TME)修正和自适应纳米簇(NCs),以介导光辅助的分级肿瘤内穿透。特别是,通过FAP-α靶向肽修饰的1 O 2敏感聚合物的自组装,制备了大尺寸的NC(〜50 nm),该聚合物进一步用于包裹结合了小分子的树枝状聚合物(〜5 nm)。 Ce6并装有DOX(DC / D)。全身给药后,NC有效靶向CAF,并产生1 O 2的致死水平。在光照射下,这会耗尽CAF,并随之使NC分解,从而释放出小型的带正电荷的DC / D。这样的基质衰减和NCs转化共同促进了DC / D传递到富含CAF的肿瘤的更深区域,其中DOX和1 O 2具有协同抗癌作用。这项研究提供了一种有效的方法,可通过同时和时空重新配置纳米特性并重塑TME来促进纳米药物在肿瘤中的渗透。











































 京公网安备 11010802027423号
京公网安备 11010802027423号