当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanodisc self-assembly is thermodynamically reversible and controllable.
Soft Matter ( IF 2.9 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0sm00336k Tyler Camp 1 , Stephen G Sligar 2
Soft Matter ( IF 2.9 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0sm00336k Tyler Camp 1 , Stephen G Sligar 2
Affiliation
|
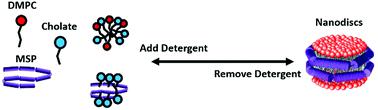
Many highly ordered complex systems form by the spontaneous self-assembly of simpler subunits. An important biophysical tool that relies on self-assembly is the Nanodisc system, which finds extensive use as native-like environments for studying membrane proteins. Nanodiscs are self-assembled from detergent-solubilized mixtures of phospholipids and engineered helical proteins called membrane scaffold proteins (MSPs). Detergent removal results in the formation of nanoscale bilayers stabilized by two MSP “belts.” Despite their numerous applications in biology, and contributions from many laboratories world-wide, little is known about the self-assembly process such as when the bilayer forms or when the MSP associates with lipids. We use fluorescence and optical spectroscopy to probe self-assembly at various equilibria defined by the detergent concentration. We show that the bilayer begins forming below the critical micellar concentration of the detergent (10 mM), and the association of MSP and lipids begins at lower detergent levels, showing a dependence on the concentrations of MSP and lipids. Following the dissolution process by adding detergent to purified Nanodiscs demonstrates that the self-assembly is reversible. Our data demonstrate that Nanodisc self-assembly is experimentally accessible, and that controlling the detergent concentration allows exquisite control over the self-assembly reaction. This improved understanding of self-assembly could lead to better functional incorporation of hitherto intractable membrane target proteins.
中文翻译:

纳米盘的自组装是热力学可逆的和可控制的。
许多高度有序的复杂系统是由更简单的亚基的自发自组装形成的。依赖自组装的重要生物物理工具是Nanodisc系统,该系统广泛用作研究膜蛋白的类似天然的环境。纳米光盘是由去污剂溶解的磷脂和工程螺旋蛋白(称为膜支架蛋白(MSP))的混合物自组装而成的。去除洗涤剂会形成由两个MSP“带”稳定的纳米级双层。尽管它们在生物学中有广泛的应用,并且在世界范围内有许多实验室的贡献,但对于自组装过程(例如双层何时形成或MSP与脂质缔合的时间)知之甚少。我们使用荧光和光谱法在洗涤剂浓度定义的各种平衡下探测自组装。我们显示双层开始形成低于清洁剂的临界胶束浓度(10 mM),并且MSP和脂质的缔合始于较低的清洁剂水平,显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。我们显示双层开始形成低于清洁剂的临界胶束浓度(10 mM),并且MSP和脂质的缔合始于较低的清洁剂水平,显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。我们显示双层开始形成低于清洁剂的临界胶束浓度(10 mM),并且MSP和脂质的缔合始于较低的清洁剂水平,显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。控制去污剂浓度可以很好地控制自组装反应。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。控制去污剂浓度可以很好地控制自组装反应。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。
更新日期:2020-06-24
中文翻译:

纳米盘的自组装是热力学可逆的和可控制的。
许多高度有序的复杂系统是由更简单的亚基的自发自组装形成的。依赖自组装的重要生物物理工具是Nanodisc系统,该系统广泛用作研究膜蛋白的类似天然的环境。纳米光盘是由去污剂溶解的磷脂和工程螺旋蛋白(称为膜支架蛋白(MSP))的混合物自组装而成的。去除洗涤剂会形成由两个MSP“带”稳定的纳米级双层。尽管它们在生物学中有广泛的应用,并且在世界范围内有许多实验室的贡献,但对于自组装过程(例如双层何时形成或MSP与脂质缔合的时间)知之甚少。我们使用荧光和光谱法在洗涤剂浓度定义的各种平衡下探测自组装。我们显示双层开始形成低于清洁剂的临界胶束浓度(10 mM),并且MSP和脂质的缔合始于较低的清洁剂水平,显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。我们显示双层开始形成低于清洁剂的临界胶束浓度(10 mM),并且MSP和脂质的缔合始于较低的清洁剂水平,显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。我们显示双层开始形成低于清洁剂的临界胶束浓度(10 mM),并且MSP和脂质的缔合始于较低的清洁剂水平,显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。显示出对MSP和脂质浓度的依赖性。在溶解过程中,通过向纯化的纳米圆盘中添加洗涤剂,证明了自组装是可逆的。我们的数据表明,纳米光盘的自组装可通过实验获得,并且控制去污剂的浓度可实现对自组装反应的精确控制。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。控制去污剂浓度可以很好地控制自组装反应。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。控制去污剂浓度可以很好地控制自组装反应。对自组装的这种更好的理解可以导致迄今难以处理的膜靶蛋白的更好的功能整合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号