当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergism of anisotropic and computational NMR methods reveals the likely configuration of phormidolide A.
Chemical Communications ( IF 4.3 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0cc03055d Ikenna E Ndukwe 1 , Xiao Wang 2 , Nelson Y S Lam 3 , Kristaps Ermanis 3 , Kelsey L Alexander 4 , Matthew J Bertin 5 , Gary E Martin 6 , Garrett Muir 7 , Ian Paterson 3 , Robert Britton 7 , Jonathan M Goodman 3 , Eric J N Helfrich 8 , Jörn Piel 8 , William H Gerwick 9 , R Thomas Williamson 10
Chemical Communications ( IF 4.3 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0cc03055d Ikenna E Ndukwe 1 , Xiao Wang 2 , Nelson Y S Lam 3 , Kristaps Ermanis 3 , Kelsey L Alexander 4 , Matthew J Bertin 5 , Gary E Martin 6 , Garrett Muir 7 , Ian Paterson 3 , Robert Britton 7 , Jonathan M Goodman 3 , Eric J N Helfrich 8 , Jörn Piel 8 , William H Gerwick 9 , R Thomas Williamson 10
Affiliation
|
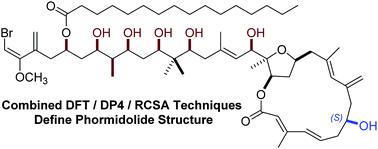
Characterization of the complex molecular scaffold of the marine polyketide natural product phormidolide A represents a challenge that has persisted for nearly two decades. In light of discordant results arising from recent synthetic and biosynthetic reports, a rigorous study of the configuration of phormidolide A was necessary. This report outlines a synergistic effort employing computational and anisotropic NMR investigation, that provided orthogonal confirmation of the reassigned side chain, as well as supporting a further correction of the C7 stereocenter.
中文翻译:

各向异性和计算 NMR 方法的协同作用揭示了 phormidolide A 的可能构型。
海洋聚酮化合物天然产物佛米多内酯 A 的复杂分子支架的表征是一个持续了近二十年的挑战。鉴于最近的合成和生物合成报告得出的不一致结果,有必要对佛米多内酯 A 的构型进行严格研究。该报告概述了采用计算和各向异性 NMR 研究的协同努力,该研究提供了重新分配的侧链的正交确认,并支持 C7 立构中心的进一步校正。
更新日期:2020-07-09
中文翻译:

各向异性和计算 NMR 方法的协同作用揭示了 phormidolide A 的可能构型。
海洋聚酮化合物天然产物佛米多内酯 A 的复杂分子支架的表征是一个持续了近二十年的挑战。鉴于最近的合成和生物合成报告得出的不一致结果,有必要对佛米多内酯 A 的构型进行严格研究。该报告概述了采用计算和各向异性 NMR 研究的协同努力,该研究提供了重新分配的侧链的正交确认,并支持 C7 立构中心的进一步校正。











































 京公网安备 11010802027423号
京公网安备 11010802027423号