当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adipic acid formation from cyclohexanediol using platinum and vanadium catalysts: elucidating the role of homogeneous vanadium species
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0cy00914h Owen Rogers 1, 2, 3, 4, 5 , Samuel Pattisson 1, 2, 3, 4, 5 , Rebecca V. Engel 1, 2, 3, 4, 5 , Robert L. Jenkins 1, 2, 3, 4, 5 , Keith Whiston 5, 6, 7, 8 , Stuart H. Taylor 1, 2, 3, 4, 5 , Graham J. Hutchings 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0cy00914h Owen Rogers 1, 2, 3, 4, 5 , Samuel Pattisson 1, 2, 3, 4, 5 , Rebecca V. Engel 1, 2, 3, 4, 5 , Robert L. Jenkins 1, 2, 3, 4, 5 , Keith Whiston 5, 6, 7, 8 , Stuart H. Taylor 1, 2, 3, 4, 5 , Graham J. Hutchings 1, 2, 3, 4, 5
Affiliation
|
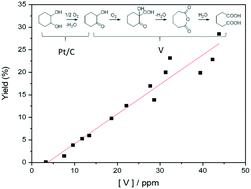
Vanadium compounds have shown great potential alongside Pt/C for the oxidation of cyclohexanediol to adipic acid. However, the low stability of these materials often leads to ambiguity when considering the homogeneous or heterogeneous nature of the active species. In this article we describe our attempts to synthesise stable vanadium catalysts through the utilisation of vanadium bronze structures. By the addition of sodium, copper or silver into these structures, leaching could be decreased to 5% for AgVO3, compared to 88.4% with V2O5. These reactions were run in aqueous conditions under 3 bar O2. However, despite significant stabilisation of vanadium in the bronze structures, we show that as little as 7.6 ppm of a homogeneous vanadium species in the reaction solution can cause the selective oxidation of 2-hydroxycyclohexanone to adipic acid. Analysis of the speciation by 51V NMR and UV-vis has revealed the active species to be in the +5 oxidation state in the form of a decavanadate compound with the presence of small amounts of monovanadate.
中文翻译:

使用铂和钒催化剂从环己二醇中形成己二酸:阐明均相钒物种的作用
钒化合物与Pt / C一起显示出将环己二醇氧化为己二酸的巨大潜力。但是,考虑到活性物质的均质或异质性质,这些材料的低稳定性通常会导致模棱两可。在本文中,我们描述了通过利用钒青铜结构合成稳定的钒催化剂的尝试。通过向这些结构中添加钠,铜或银,AgVO 3的浸出率可降低至5%,而V 2 O 5则为88.4%。这些反应在3 bar O 2的水性条件下进行。然而,尽管钒在青铜结构中具有显着的稳定性,但我们发现,反应溶液中低至7.6 ppm的均相钒物种会导致2-羟基环己酮选择性氧化为己二酸。通过51 V NMR和UV-vis对形态的分析显示,活性物质以十钒酸盐化合物的形式处于+5氧化态,并且存在少量的单钒酸盐。
更新日期:2020-07-06
中文翻译:

使用铂和钒催化剂从环己二醇中形成己二酸:阐明均相钒物种的作用
钒化合物与Pt / C一起显示出将环己二醇氧化为己二酸的巨大潜力。但是,考虑到活性物质的均质或异质性质,这些材料的低稳定性通常会导致模棱两可。在本文中,我们描述了通过利用钒青铜结构合成稳定的钒催化剂的尝试。通过向这些结构中添加钠,铜或银,AgVO 3的浸出率可降低至5%,而V 2 O 5则为88.4%。这些反应在3 bar O 2的水性条件下进行。然而,尽管钒在青铜结构中具有显着的稳定性,但我们发现,反应溶液中低至7.6 ppm的均相钒物种会导致2-羟基环己酮选择性氧化为己二酸。通过51 V NMR和UV-vis对形态的分析显示,活性物质以十钒酸盐化合物的形式处于+5氧化态,并且存在少量的单钒酸盐。











































 京公网安备 11010802027423号
京公网安备 11010802027423号