当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Stabilization of Aspartic Acid Protonation State within a Given Protein Conformation Occurs via Specific "Molecular Association".
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.jpcb.0c02629 Debashree Bandyopadhyay 1 , Akshay Bhatnagar 1 , Shobhit Jain 1 , Prabhav Pratyaksh 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.jpcb.0c02629 Debashree Bandyopadhyay 1 , Akshay Bhatnagar 1 , Shobhit Jain 1 , Prabhav Pratyaksh 1
Affiliation
|
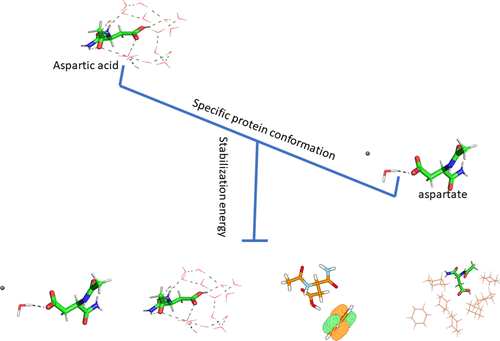
Proteins involved in proton-/electron-transfer processes often possess “functional” aspartates/aspartic acids (Asp) with variable protonation states. The mechanism of Asp protonation–deprotonation within proteins is unclear. Two questions were asked—the possible types of determinants responsible for Asp protonation–deprotonation and the spatial arrangements of the determinants leading to selective stabilization. The questions were analyzed using nine different solvent models, which scanned the complete protein dielectric range, and four protein models, which illustrated the spatial arrangements around Asp, termed as “molecular association”. The methods employed were quantum chemical calculations and constant pH simulations. The types of the determinants identified were charge–charge interaction, H bonding, dipole−π interaction, extended electronic conjugation, dielectric effect, and solvent accessibility. All solvent-exposed Asp [buried fraction (BF) less than 0.5] were aspartates, and buried Asp were either aspartic acids or aspartates, each having a different “molecular association”. The exposed aspartates were stabilized via a H-bonding network with bulk water, buried aspartates via salt bridge or, minimum, two intramolecular H bonds, and buried aspartic acids via, minimum, one intramolecular H bond. An “acid–alcohol pair” (involving Ser/Thr/Tyr) was a common determinant to any “functional” buried aspartate/aspartic acid. Higher energy “molecular associations” observed within proteins compared to those within water, presumably, indicated easy molecular restructuring and alteration of the Asp protonation states during a protein-mediated proton/electron transfer.
中文翻译:

给定蛋白质构象中的天冬氨酸质子化状态的选择性稳定通过特定的“分子缔合”发生。
质子/电子转移过程中涉及的蛋白质通常具有质子化状态可变的“功能性”天冬氨酸/天冬氨酸(Asp)。蛋白中Asp质子化-去质子化的机制尚不清楚。提出了两个问题-可能引起Asp质子化的决定因素类型-去质子化以及决定因素的空间排列导致选择性稳定。使用九个不同的溶剂模型(扫描了整个蛋白质的介电范围)和四个蛋白质模型(用于说明Asp周围的空间排列,称为“分子缔合”)分析了问题。所采用的方法是量子化学计算和恒定pH模拟。确定的行列式的类型为电荷-电荷相互作用,H键,偶极-π相互作用,扩展的电子共轭,介电效应和溶剂可及性。所有溶剂暴露的Asp [埋入分数(BF)小于0.5]是天冬氨酸,而埋藏的Asp是天冬氨酸或天冬氨酸,各自具有不同的“分子缔合”。暴露的天冬氨酸通过与大量水的H键网络稳定,通过盐桥或至少2个分子内H键掩埋天冬氨酸,通过至少1个分子内H键掩埋天冬氨酸。“酸-醇对”(涉及Ser / Thr / Tyr)是任何“功能性”埋藏的天冬氨酸/天冬氨酸的共同决定因素。与在水中相比,在蛋白质内观察到的较高能量“分子缔合”推测表明在蛋白质介导的质子/电子转移过程中,分子易于重构和Asp质子化状态改变。
更新日期:2020-07-02
中文翻译:

给定蛋白质构象中的天冬氨酸质子化状态的选择性稳定通过特定的“分子缔合”发生。
质子/电子转移过程中涉及的蛋白质通常具有质子化状态可变的“功能性”天冬氨酸/天冬氨酸(Asp)。蛋白中Asp质子化-去质子化的机制尚不清楚。提出了两个问题-可能引起Asp质子化的决定因素类型-去质子化以及决定因素的空间排列导致选择性稳定。使用九个不同的溶剂模型(扫描了整个蛋白质的介电范围)和四个蛋白质模型(用于说明Asp周围的空间排列,称为“分子缔合”)分析了问题。所采用的方法是量子化学计算和恒定pH模拟。确定的行列式的类型为电荷-电荷相互作用,H键,偶极-π相互作用,扩展的电子共轭,介电效应和溶剂可及性。所有溶剂暴露的Asp [埋入分数(BF)小于0.5]是天冬氨酸,而埋藏的Asp是天冬氨酸或天冬氨酸,各自具有不同的“分子缔合”。暴露的天冬氨酸通过与大量水的H键网络稳定,通过盐桥或至少2个分子内H键掩埋天冬氨酸,通过至少1个分子内H键掩埋天冬氨酸。“酸-醇对”(涉及Ser / Thr / Tyr)是任何“功能性”埋藏的天冬氨酸/天冬氨酸的共同决定因素。与在水中相比,在蛋白质内观察到的较高能量“分子缔合”推测表明在蛋白质介导的质子/电子转移过程中,分子易于重构和Asp质子化状态改变。









































 京公网安备 11010802027423号
京公网安备 11010802027423号