当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interplay between Thermodynamics and Kinetics on Polymorphic Behavior of Vortioxetine Hydrobromide in Reactive Crystallization
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.oprd.9b00384 Yun Cao 1, 2 , Shichao Du 1, 2 , Xiao Ke 3 , Shijie Xu 4 , Yangshan Lan 3 , Teng Zhang 1, 2 , Weiwei Tang 1, 2 , Jingkang Wang 1, 2 , Junbo Gong 1, 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.oprd.9b00384 Yun Cao 1, 2 , Shichao Du 1, 2 , Xiao Ke 3 , Shijie Xu 4 , Yangshan Lan 3 , Teng Zhang 1, 2 , Weiwei Tang 1, 2 , Jingkang Wang 1, 2 , Junbo Gong 1, 2
Affiliation
|
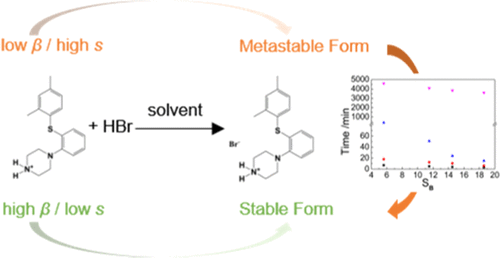
Reactive crystallization (RC) of vortioxetine hydrobromide (VH) in four solvents was investigated in this work. In solvents with strong hydrogen bond acceptor capacity, the thermodynamics stable form B is preferred and the conversion rate from form A to form B increases. Meanwhile, supersaturation (S) is also an important factor affecting the polymorphic behavior. When supersaturation is high, the metastable form A is preferred, while the conversion rate also increases. Solubility data and nucleation rate were adopted to explain thermodynamic and kinetic reasons for solvent and supersaturation effects here. In addition, RC process in the four solvents at 313.15 K (SB ≈ 5.60) was detected by online attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectroscopy and offline power X-ray diffraction (PXRD). Results show that the effect of reaction kinetics on polymorph formation of VH can be ignored and nucleation and growth of form B is the controlling step of subsequent suspension polymorphic conversion process.
中文翻译:

反应动力学结晶中氢动力学的动力学和动力学相互作用对伏立西汀氢溴酸盐多晶型行为的影响
这项工作研究了伏罗西汀氢溴酸盐(VH)在四种溶剂中的反应结晶(RC)。在具有强氢键受体能力的溶剂中,优选热力学稳定的形式B,并且从形式A到形式B的转化率提高。同时,过饱和度(S)也是影响多态行为的重要因素。当过饱和度高时,亚稳态形式A是优选的,同时转化率也增加。本文采用溶解度数据和成核速率解释溶剂和过饱和作用的热力学和动力学原因。此外,在313.15 K(S B在线衰减全反射-傅立叶变换红外(ATR-FTIR)光谱和离线功率X射线衍射(PXRD)检测到≈5.60)。结果表明,反应动力学对VH多晶型形成的影响可以忽略,而晶型B的成核和生长是后续悬浮多晶型转化过程的控制步骤。
更新日期:2020-07-17
中文翻译:

反应动力学结晶中氢动力学的动力学和动力学相互作用对伏立西汀氢溴酸盐多晶型行为的影响
这项工作研究了伏罗西汀氢溴酸盐(VH)在四种溶剂中的反应结晶(RC)。在具有强氢键受体能力的溶剂中,优选热力学稳定的形式B,并且从形式A到形式B的转化率提高。同时,过饱和度(S)也是影响多态行为的重要因素。当过饱和度高时,亚稳态形式A是优选的,同时转化率也增加。本文采用溶解度数据和成核速率解释溶剂和过饱和作用的热力学和动力学原因。此外,在313.15 K(S B在线衰减全反射-傅立叶变换红外(ATR-FTIR)光谱和离线功率X射线衍射(PXRD)检测到≈5.60)。结果表明,反应动力学对VH多晶型形成的影响可以忽略,而晶型B的成核和生长是后续悬浮多晶型转化过程的控制步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号