当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituted 4,5'-Bithiazoles as Catalytic Inhibitors of Human DNA Topoisomerase IIα.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.jcim.0c00202 Kaja Bergant Loboda 1, 2 , Matej Janežič 3 , Martina Štampar 4 , Bojana Žegura 4 , Metka Filipič 4 , Andrej Perdih 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.jcim.0c00202 Kaja Bergant Loboda 1, 2 , Matej Janežič 3 , Martina Štampar 4 , Bojana Žegura 4 , Metka Filipič 4 , Andrej Perdih 1
Affiliation
|
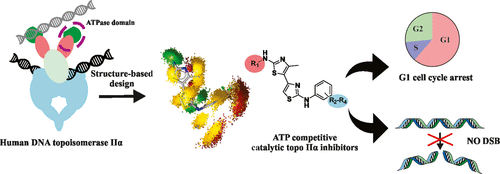
Human type II topoisomerases, molecular motors that alter the DNA topology, are a major target of modern chemotherapy. Groups of catalytic inhibitors represent a new approach to overcome the known limitations of topoisomerase II poisons such as cardiotoxicity and induction of secondary tumors. Here, we present a class of substituted 4,5′-bithiazoles as catalytic inhibitors targeting the human DNA topoisomerase IIα. Based on a structural comparison of the ATPase domains of human and bacterial type II topoisomerase, a focused chemical library of 4,5′-bithiazoles was assembled and screened to identify compounds that better fit the topology of the human topo IIα adenosine 5′-triphosphate (ATP) binding site. Selected compounds showed inhibition of human topo IIα comparable to that of the etoposide topo II drug, revealing a new class of inhibitors targeting this molecular motor. Further investigations showed that compounds act as catalytic inhibitors via competitive ATP inhibition. We also confirmed binding to the truncated ATPase domain of topo IIα and modeled the inhibitor molecular recognition with molecular simulations and dynophore models. The compounds also displayed promising cytotoxicity against HepG2 and MCF-7 cell lines comparable to that of etoposide. In a more detailed study with the HepG2 cell line, there was no induction of DNA double-strand breaks (DSBs), and the compounds were able to reduce cell proliferation and stop the cell cycle mainly in the G1 phase. This confirms the mechanism of action of these compounds, which differs from topo II poisons also at the cellular level. Substituted 4,5′-bithiazoles appear to be a promising class for further development toward efficient and potentially safer cancer therapies exploiting the alternative topo II inhibition paradigm.
中文翻译:

取代4,5'-Bithiazoles作为人类DNA拓扑异构酶IIα的催化抑制剂。
人类II型拓扑异构酶是改变DNA拓扑结构的分子马达,是现代化学疗法的主要目标。催化抑制剂的组代表了一种新的方法,可以克服拓扑异构酶II毒物的已知局限性,例如心脏毒性和继发性肿瘤的诱导。在这里,我们提出了一类取代的4,5'-噻唑类化合物作为针对人类DNA拓扑异构酶IIα的催化抑制剂。根据人和细菌II型拓扑异构酶ATPase结构域的结构比较,组装并筛选了4,5'-联噻唑的重点化学文库,以鉴定出更适合人拓扑IIα腺苷5'-三磷酸拓扑结构的化合物(ATP)结合位点。所选化合物对人topoIIα的抑制作用与依托泊苷topo II药物相当,揭示了针对这种分子马达的新型抑制剂。进一步的研究表明,化合物通过竞争性ATP抑制作用起催化抑制剂的作用。我们还证实了与topoIIα截短的ATPase结构域的结合,并通过分子模拟和测力计模型对抑制剂的分子识别进行了建模。该化合物还显示出与依托泊苷相当的针对HepG2和MCF-7细胞系的有希望的细胞毒性。在有关HepG2细胞系的更详细研究中,没有诱导DNA双链断裂(DSB),并且这些化合物能够减少细胞增殖并主要在G1期停止细胞周期。这证实了这些化合物的作用机理,在细胞水平上也不同于topo II毒物。替代4
更新日期:2020-07-27
中文翻译:

取代4,5'-Bithiazoles作为人类DNA拓扑异构酶IIα的催化抑制剂。
人类II型拓扑异构酶是改变DNA拓扑结构的分子马达,是现代化学疗法的主要目标。催化抑制剂的组代表了一种新的方法,可以克服拓扑异构酶II毒物的已知局限性,例如心脏毒性和继发性肿瘤的诱导。在这里,我们提出了一类取代的4,5'-噻唑类化合物作为针对人类DNA拓扑异构酶IIα的催化抑制剂。根据人和细菌II型拓扑异构酶ATPase结构域的结构比较,组装并筛选了4,5'-联噻唑的重点化学文库,以鉴定出更适合人拓扑IIα腺苷5'-三磷酸拓扑结构的化合物(ATP)结合位点。所选化合物对人topoIIα的抑制作用与依托泊苷topo II药物相当,揭示了针对这种分子马达的新型抑制剂。进一步的研究表明,化合物通过竞争性ATP抑制作用起催化抑制剂的作用。我们还证实了与topoIIα截短的ATPase结构域的结合,并通过分子模拟和测力计模型对抑制剂的分子识别进行了建模。该化合物还显示出与依托泊苷相当的针对HepG2和MCF-7细胞系的有希望的细胞毒性。在有关HepG2细胞系的更详细研究中,没有诱导DNA双链断裂(DSB),并且这些化合物能够减少细胞增殖并主要在G1期停止细胞周期。这证实了这些化合物的作用机理,在细胞水平上也不同于topo II毒物。替代4









































 京公网安备 11010802027423号
京公网安备 11010802027423号